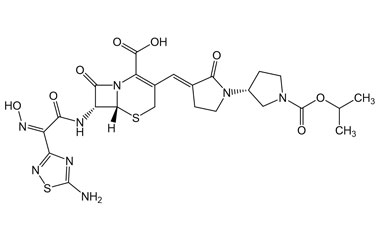
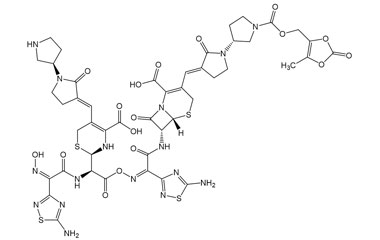
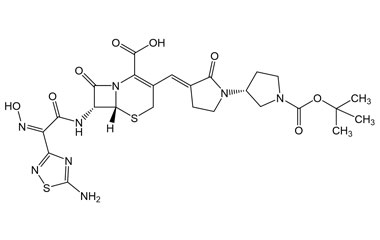
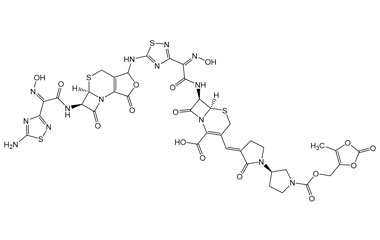
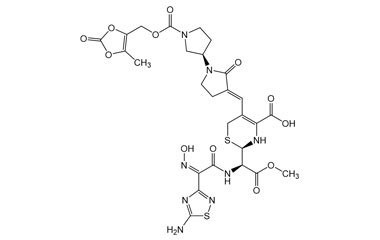
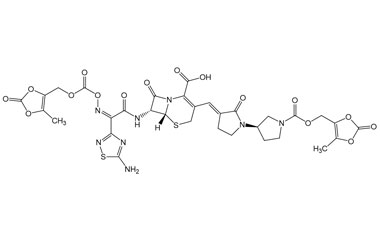
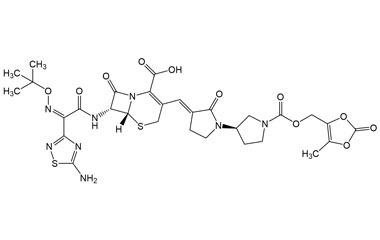
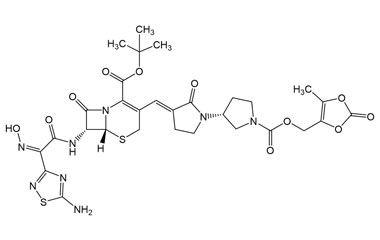
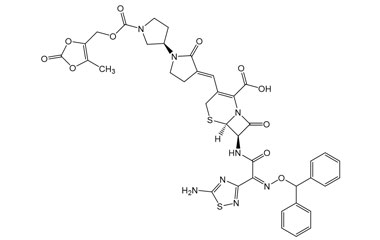
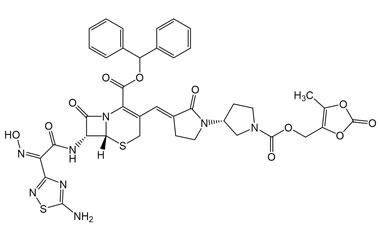
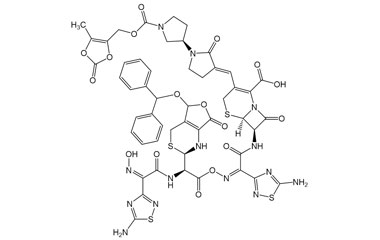
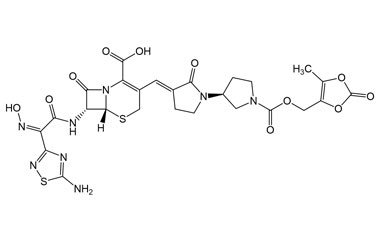
Ceftobiprole
Metabolites
- Ceftobiprole is the first cephalosporin to demonstrate clinical efficacy in patients with infections due to methicillin-resistant staphylococci and, if approved by regulatory authorities, is expected to be a useful addition to the armamentarium of agents for the treatment of complicated skin infections and pneumonia. Reference standards of Ceftobiprole API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
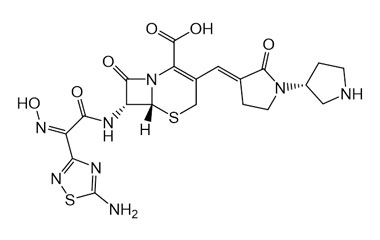
[pname] => Ceftobiprole
[catalogue_number] => PA 03 3820000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 534.57
[form] => C20H22N8O6S2
[cas] => 209467-52-7
[pslug] => 209467-52-7-ceftobiprole-api-pa033820000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 03 3820000
Molecular Formula : C20H22N8O6S2
Molecular Weight : 534.57
stdClass Object
(
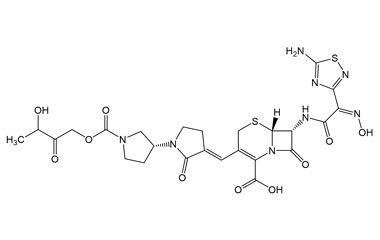
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((3'R)-1'-((3-hydroxy-2-oxobutoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 664.67
[form] => C25H28N8O10S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-3r-1-3-hydroxy-2-oxobutoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((3'R)-1'-((3-hydroxy-2-oxobutoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821000
Molecular Formula : C25H28N8O10S2
Molecular Weight : 664.67
stdClass Object
(
[pname] => (6R,7R)-7-((E)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821001
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 690.66
[form] => C26H26N8O11S2
[cas] => NA
[pslug] => 6r-7r-7-e-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821001
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
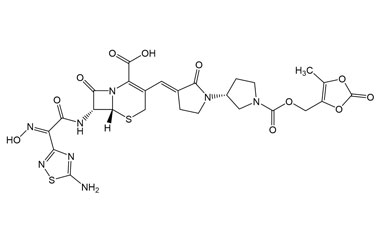
(6R,7R)-7-((E)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821001
Molecular Formula : C26H26N8O11S2
Molecular Weight : 690.66
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((Z)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821002
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 690.66
[form] => C26H26N8O11S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-z-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821002
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
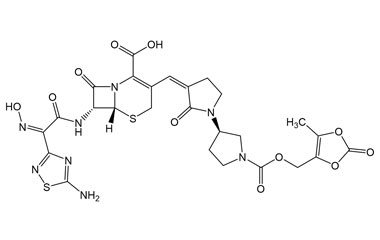
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((Z)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821002
Molecular Formula : C26H26N8O11S2
Molecular Weight : 690.66
stdClass Object
(
[pname] => (R)-2-((R)-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)(carboxy)methyl)-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
[catalogue_number] => PA 03 3821003
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 708.67
[form] => C26H28N8O12S2
[cas] => NA
[pslug] => r-2-r-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-carboxy-methyl-5-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazine-4-carboxylic-acid-pa033821003
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
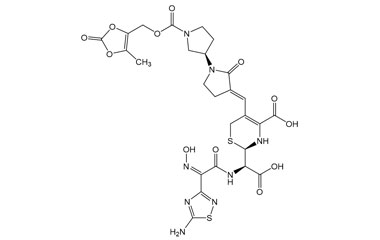
(R)-2-((R)-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)(carboxy)methyl)-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
Catalogue No.:PA 03 3821003
Molecular Formula : C26H28N8O12S2
Molecular Weight : 708.67
stdClass Object
(
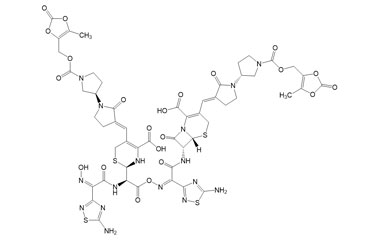
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821004
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 1381.32
[form] => C52H52N16O22S4
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-r-2-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-2-r-4-carboxy-5-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazin-2-yl-acetoxy-imino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821004
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
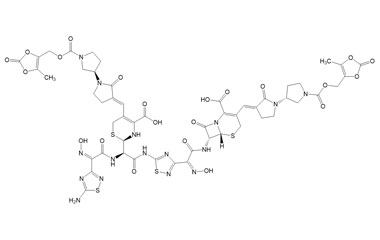
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821004
Molecular Formula : C52H52N16O22S4
Molecular Weight : 1381.32
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-((R)-2-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetamido)-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821005
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 1381.32
[form] => C52H52N16O22S4
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-r-2-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-2-r-4-carboxy-5-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazin-2-yl-acetamido-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821005
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-((R)-2-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetamido)-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821005
Molecular Formula : C52H52N16O22S4
Molecular Weight : 1381.32
stdClass Object
(
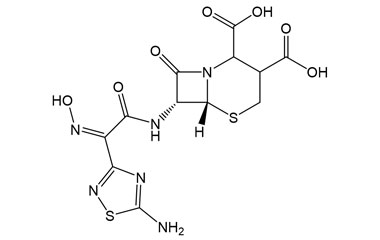
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]octane-2,3-dicarboxylic Acid
[catalogue_number] => PA 03 3821006
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 416.38
[form] => C12H12N6O7S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-8-oxo-5-thia-1-azabicyclo-4-2-0-octane-2-3-dicarboxylic-acid-pa033821006
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]octane-2,3-dicarboxylic Acid
Catalogue No.:PA 03 3821006
Molecular Formula : C12H12N6O7S2
Molecular Weight : 416.38
stdClass Object
(
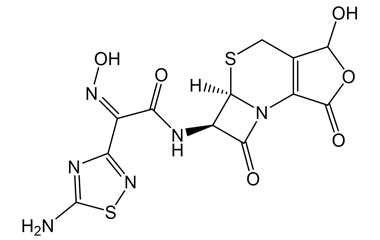
[pname] => (Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-N-((5aR,6R)-3-hydroxy-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl)-2-(hydroxyimino)acetamide
[catalogue_number] => PA 03 3821007
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 398.37
[form] => C12H10N6O6S2
[cas] => NA
[pslug] => z-2-5-amino-1-2-4-thiadiazol-3-yl-n-5ar-6r-3-hydroxy-1-7-dioxo-1-4-5a-6-tetrahydro-3h-7h-azeto-2-1-b-furo-3-4-d-1-3-thiazin-6-yl-2-hydroxyimino-acetamide-pa033821007
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-N-((5aR,6R)-3-hydroxy-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl)-2-(hydroxyimino)acetamide
Catalogue No.:PA 03 3821007
Molecular Formula : C12H10N6O6S2
Molecular Weight : 398.37
stdClass Object
(
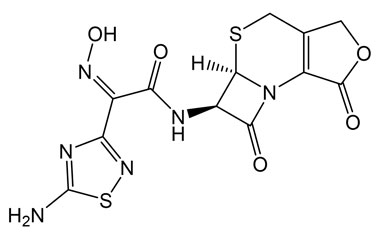
[pname] => (Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-N-((5aR,6R)-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl)-2-(hydroxyimino)acetamide
[catalogue_number] => PA 03 3821008
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 382.37
[form] => C12H10N6O5S2
[cas] => NA
[pslug] => z-2-5-amino-1-2-4-thiadiazol-3-yl-n-5ar-6r-1-7-dioxo-1-4-5a-6-tetrahydro-3h-7h-azeto-2-1-b-furo-3-4-d-1-3-thiazin-6-yl-2-hydroxyimino-acetamide-pa033821008
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-N-((5aR,6R)-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl)-2-(hydroxyimino)acetamide
Catalogue No.:PA 03 3821008
Molecular Formula : C12H10N6O5S2
Molecular Weight : 382.37
stdClass Object
(
[pname] => (R)-2-((R)-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)(carboxy)methyl)-5-((E)-((R)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
[catalogue_number] => PA 03 3821009
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 552.58
[form] => C20H24N8O7S2
[cas] => NA
[pslug] => r-2-r-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-carboxy-methyl-5-e-r-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazine-4-carboxylic-acid-pa033821009
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
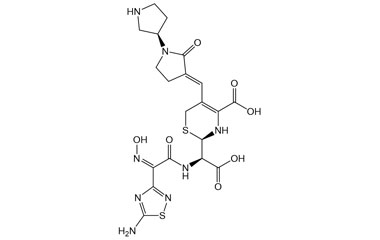
(R)-2-((R)-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)(carboxy)methyl)-5-((E)-((R)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
Catalogue No.:PA 03 3821009
Molecular Formula : C20H24N8O7S2
Molecular Weight : 552.58
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-8-oxo-3-((E)-(2-oxo-1-((R)-tetrahydrofuran-3-yl)pyrrolidin-3-ylidene)methyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821010
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 535.55
[form] => C20H21N7O7S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-8-oxo-3-e-2-oxo-1-r-tetrahydrofuran-3-yl-pyrrolidin-3-ylidene-methyl-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821010
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
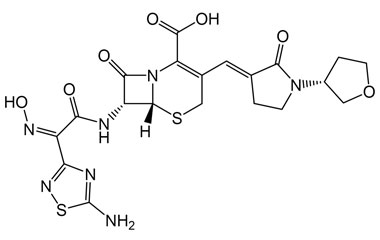
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-8-oxo-3-((E)-(2-oxo-1-((R)-tetrahydrofuran-3-yl)pyrrolidin-3-ylidene)methyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821010
Molecular Formula : C20H21N7O7S2
Molecular Weight : 535.55
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(isopropoxycarbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821011
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 620.66
[form] => C24H28N8O8S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-isopropoxycarbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821011
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(isopropoxycarbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821011
Molecular Formula : C24H28N8O8S2
Molecular Weight : 620.66
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821012
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 1225.23
[form] => C46H48N16O17S4
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-r-2-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-2-r-4-carboxy-5-e-r-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazin-2-yl-acetoxy-imino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821012
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((R)-4-carboxy-5-((E)-((R)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821012
Molecular Formula : C46H48N16O17S4
Molecular Weight : 1225.23
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(tert-butoxycarbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821013
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 634.68
[form] => C25H30N8O8S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-tert-butoxycarbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821013
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(tert-butoxycarbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821013
Molecular Formula : C25H30N8O8S2
Molecular Weight : 634.68
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-(((5aR,6R)-6-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-3-yl)amino)-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821014
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 1071.01
[form] => C38H34N14O16S4
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-5ar-6r-6-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-1-7-dioxo-1-4-5a-6-tetrahydro-3h-7h-azeto-2-1-b-furo-3-4-d-1-3-thiazin-3-yl-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821014
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-(((5aR,6R)-6-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-1,7-dioxo-1,4,5a,6-tetrahydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-3-yl)amino)-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821014
Molecular Formula : C38H34N14O16S4
Molecular Weight : 1071.01
stdClass Object
(
[pname] => (R)-2-((R)-1-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-methoxy-2-oxoethyl)-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
[catalogue_number] => PA 03 3821015
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 722.7
[form] => C27H30N8O12S2
[cas] => NA
[pslug] => r-2-r-1-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-2-methoxy-2-oxoethyl-5-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-3-6-dihydro-2h-1-3-thiazine-4-carboxylic-acid-pa033821015
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(R)-2-((R)-1-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-methoxy-2-oxoethyl)-5-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-3,6-dihydro-2H-1,3-thiazine-4-carboxylic Acid
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)oxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821016
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 846.75
[form] => C32H30N8O16S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-oxy-imino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821016
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)oxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821016
Molecular Formula : C32H30N8O16S2
Molecular Weight : 846.75
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(tert-butoxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821017
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 746.77
[form] => C30H34N8O11S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-tert-butoxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821017
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(tert-butoxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821017
Molecular Formula : C30H34N8O11S2
Molecular Weight : 746.77
stdClass Object
(
[pname] => tertButyl (6R,7R)-7-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
[catalogue_number] => PA 03 3821018
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 746.77
[form] => C30H34N8O11S2
[cas] => NA
[pslug] => tertbutyl-6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylate-pa033821018
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
tertButyl (6R,7R)-7-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
Catalogue No.:PA 03 3821018
Molecular Formula : C30H34N8O11S2
Molecular Weight : 746.77
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-((benzhydryloxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821019
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 856.88
[form] => C39H36N8O11S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-benzhydryloxy-imino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821019
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-((benzhydryloxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821019
Molecular Formula : C39H36N8O11S2
Molecular Weight : 856.88
stdClass Object
(
[pname] => Benzhydryl (6R,7R)-7-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
[catalogue_number] => PA 03 3821020
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 856.88
[form] => C39H36N8O11S2
[cas] => NA
[pslug] => benzhydryl-6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylate-pa033821020
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Benzhydryl (6R,7R)-7-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
Catalogue No.:PA 03 3821020
Molecular Formula : C39H36N8O11S2
Molecular Weight : 856.88
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((2R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((2R)-5-(benzhydryloxy)-7-oxo-1,2,5,7-tetrahydro-4H-furo[3,4-d][1,3]thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821021
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 1255.25
[form] => C51H46N14O17S4
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-2r-2-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-2-2r-5-benzhydryloxy-7-oxo-1-2-5-7-tetrahydro-4h-furo-3-4-d-1-3-thiazin-2-yl-acetoxy-imino-acetamido-3-e-r-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821021
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(((2R)-2-((Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-2-((2R)-5-(benzhydryloxy)-7-oxo-1,2,5,7-tetrahydro-4H-furo[3,4-d][1,3]thiazin-2-yl)acetoxy)imino)acetamido)-3-((E)-((R)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821021
Molecular Formula : C51H46N14O17S4
Molecular Weight : 1255.25
stdClass Object
(
[pname] => (6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((S)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
[catalogue_number] => PA 03 3821022
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 690.66
[form] => C26H26N8O11S2
[cas] => NA
[pslug] => 6r-7r-7-z-2-5-amino-1-2-4-thiadiazol-3-yl-2-hydroxyimino-acetamido-3-e-s-1-5-methyl-2-oxo-1-3-dioxol-4-yl-methoxy-carbonyl-2-oxo-1-3-bipyrrolidin-3-ylidene-methyl-8-oxo-5-thia-1-azabicyclo-4-2-0-oct-2-ene-2-carboxylic-acid-pa033821022
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(6R,7R)-7-((Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(hydroxyimino)acetamido)-3-((E)-((S)-1'-(((5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy)carbonyl)-2-oxo-[1,3'-bipyrrolidin]-3-ylidene)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
Catalogue No.:PA 03 3821022
Molecular Formula : C26H26N8O11S2
Molecular Weight : 690.66