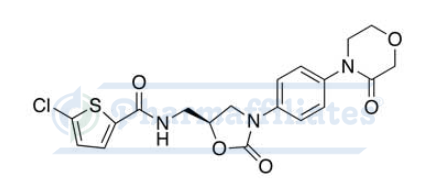
Pharmaffiliates offers Rivaroxaban API Standards (CAS 366789-02-8), a high-purity pharmaceutical ingredient used extensively in the research and development of anticoagulants. This active pharmaceutical ingredient (API) is crucial in the formulation of rivaroxaban, which prevents blood clots and reduces the risk of stroke.
Applications
Rivaroxaban plays a significant role in the development of anticoagulant therapies. Its key applications include:
- Anticoagulant Research: Used in the synthesis and formulation of blood-thinning medications.
- Stroke Prevention: Essential in preventing strokes in atrial fibrillation patients.
- Blood Clot Prevention: Used to prevent and treat deep vein thrombosis (DVT) and pulmonary embolism (PE).
Quality & Compliance
Pharmaffiliates ensures stringent quality controls for all batches of Rivaroxaban API. Each product is supplied with:
- Certificate of Analysis (COA)
- Material Safety Data Sheet (MSDS)
- Spectral Data – NMR, HPLC, LC-MS (available on request)
Disclaimer
The PASL product information for any accuracy or completeness is as per the existing knowledge furnished during the creation of the webpage.
The purchaser, user or importer of record is responsible for determining the accuracy of items at the time of ordering, as it may change without notice as per current PASL product specification.






