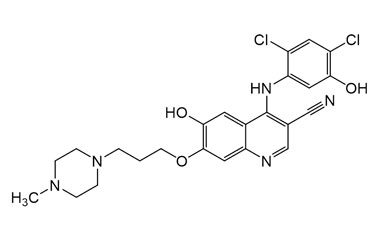
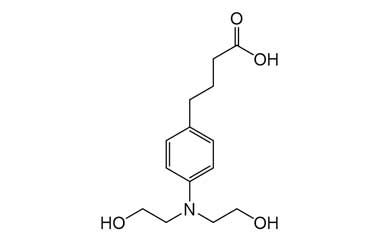
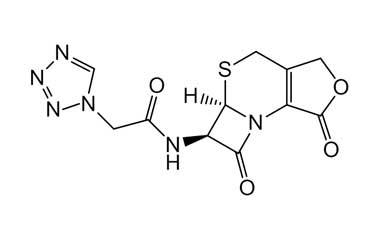
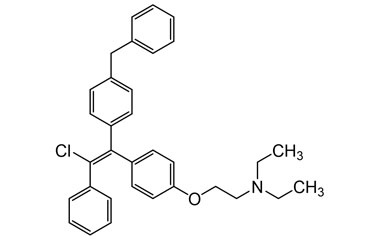
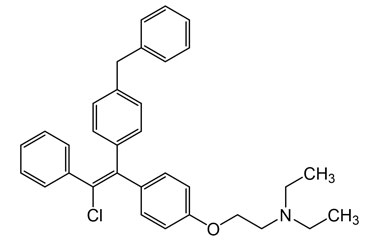
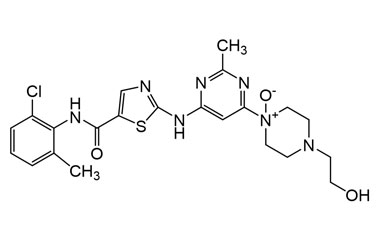
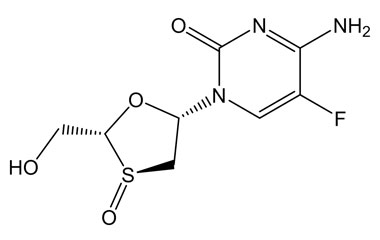
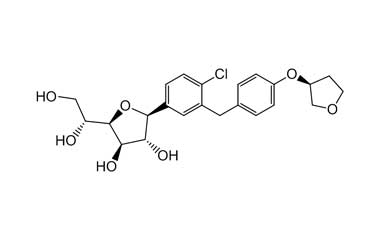
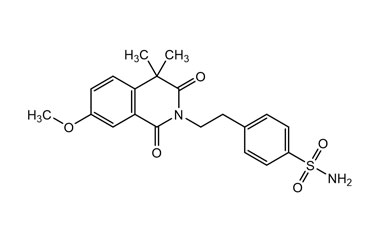
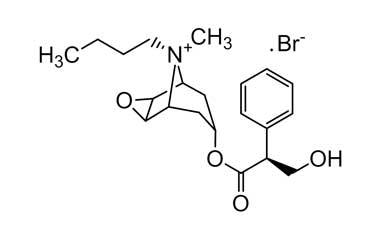
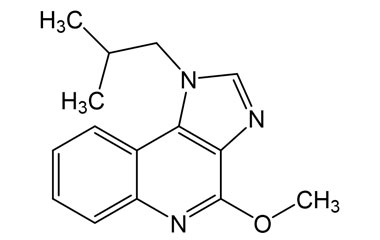
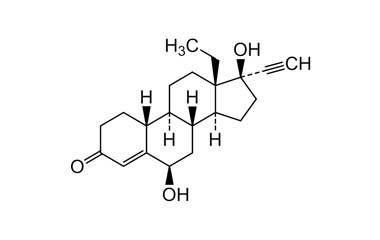
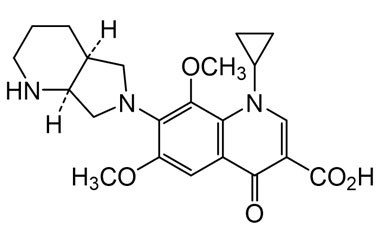
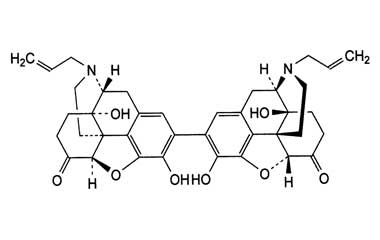
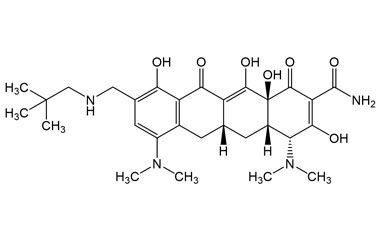
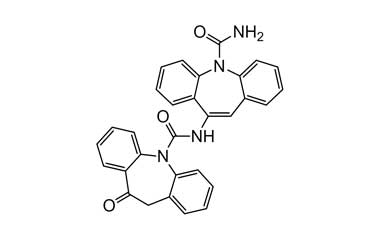
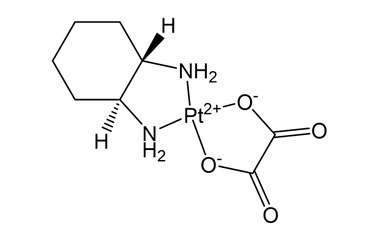
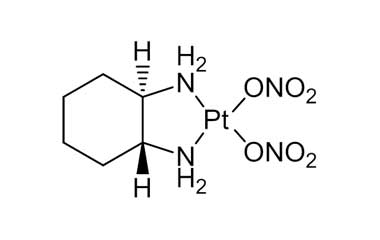
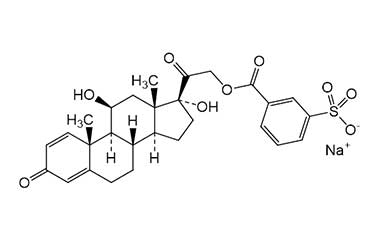
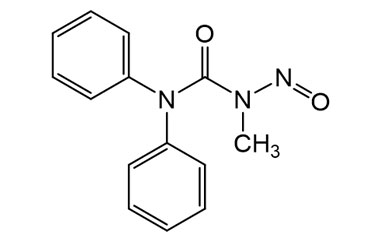
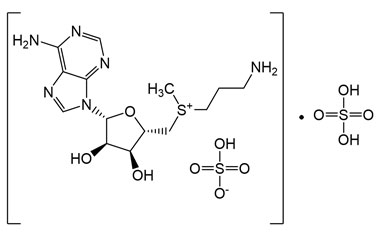
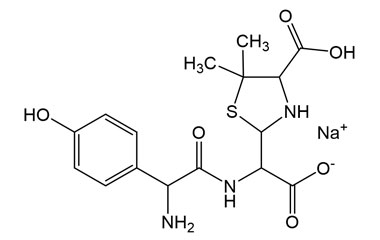
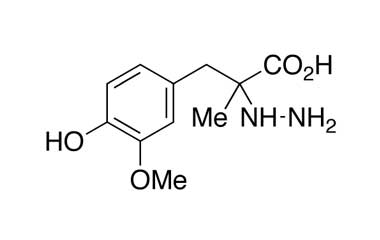
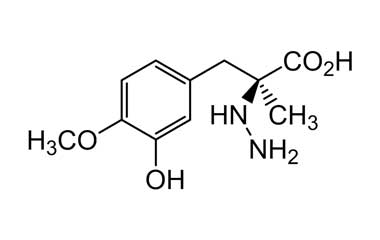
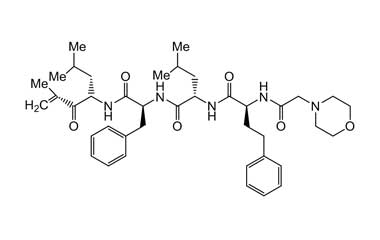
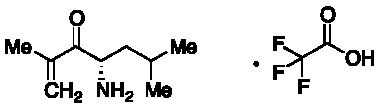
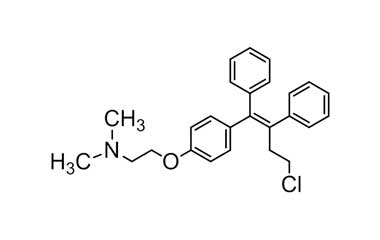
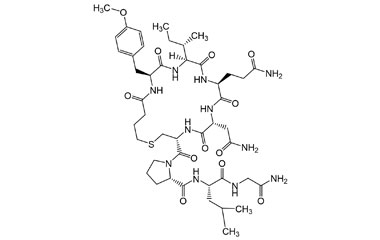
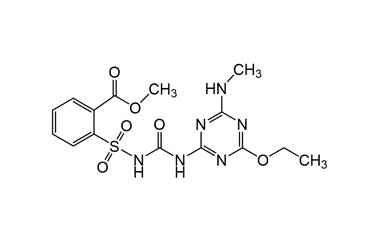
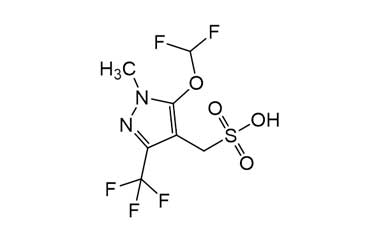
Enabling end-to-end support for
drug discovery and development
Wherever you are in your drug development journey, we offer a comprehensive
portfolio of products, models, services, and expert guidance—customized to meet your unique requirements.

Custom Capabilities
Pharmaffiliates encompass a wide range of advanced pharmaceutical services tailored to support complex R&D needs. We specialize in the synthesis of both small and large molecules, including APIs, key intermediates, chiral standards, metabolites, glucuronides, and stable isotopes. Our expertise in deuterated compounds enables applications in reaction mechanism studies, biosynthetic pathway analysis, and improved metabolic stability. We also develop novel drug delivery systems with applications in dermatology, cosmetics, and nutraceuticals. Additionally, we offer impurity profiling and polymorphism studies to ensure product quality and regulatory compliance.

Analytical Capabilities
Pharmaffiliates support comprehensive method development and validation for APIs, formulations products, and their containers-ensuring specificity, linearity, accuracy, precision, and robustness. Method transfer is supported seamlessly across laboratories. Stability studies include R&D preliminary testing, QC-based stability assessments, release and comparator studies, and photostability evaluations. Structure elucidation is carried out using advanced techniques such as UV, HPLC, UPLC, FTIR, ICP, ICP-MS, chiral chromatography, and 1D/2D NMR, delivering reliable characterization and regulatory-compliant data.

Regulatory Services
Pharmaffiliates provide comprehensive regulatory services to support pharmaceutical research, including expert consulting, dossier preparation, and DMF/ANDA submissions. Our scientifically driven approach ensures global regulatory compliance, expedites approvals, and facilitates successful clinical and bioequivalence studies through accurate, research-focused documentation and strategic guidance.

Analytical Testing
Supporting research with precision, our analytical testing services cover everything from structural identification using IR, NMR, and Mass Spectrometry to detailed purity and assay assessments. With advanced techniques like 2D NMR and on-demand precision testing, we deliver the reliable data scientists need to drive discovery and development forward.

Training & Audits
Expert-led GMP/GLP training and independent third-party audits enhance pharmaceutical quality, compliance, and operational excellence. With over 60 training programs and 110 audits completed, these services empower professionals with practical skills and ensure alignment with global regulatory standards—supporting safer, research-driven pharmaceutical development and manufacturing.

Other
We provide advanced lab solutions, FTE-based services, and specialist consulting to assist your study. Under the direction of seasoned Ph.D. scientists, our team guarantees confidentiality, open communication, and dependable outcomes. Help us to meet world standards and confidently advance your research by producing efficient, cost-effective results.

Custom Capabilities
Pharmaffiliates encompass a wide range of advanced pharmaceutical services tailored to support complex R&D needs. We specialize in the synthesis of both small and large molecules, including APIs, key intermediates, chiral standards, metabolites, glucuronides, and stable isotopes. Our expertise in deuterated compounds enables applications in reaction mechanism studies, biosynthetic pathway analysis, and improved metabolic stability. We also develop novel drug delivery systems with applications in dermatology, cosmetics, and nutraceuticals. Additionally, we offer impurity profiling and polymorphism studies to ensure product quality and regulatory compliance.

Analytical Capabilities
Pharmaffiliates support comprehensive method development and validation for APIs, formulations products, and their containers-ensuring specificity, linearity, accuracy, precision, and robustness. Method transfer is supported seamlessly across laboratories. Stability studies include R&D preliminary testing, QC-based stability assessments, release and comparator studies, and photostability evaluations. Structure elucidation is carried out using advanced techniques such as UV, HPLC, UPLC, FTIR, ICP, ICP-MS, chiral chromatography, and 1D/2D NMR, delivering reliable characterization and regulatory-compliant data.

Regulatory Services
Pharmaffiliates provide comprehensive regulatory services to support pharmaceutical research, including expert consulting, dossier preparation, and DMF/ANDA submissions. Our scientifically driven approach ensures global regulatory compliance, expedites approvals, and facilitates successful clinical and bioequivalence studies through accurate, research-focused documentation and strategic guidance.

Analytical Testing
Supporting research with precision, our analytical testing services cover everything from structural identification using IR, NMR, and Mass Spectrometry to detailed purity and assay assessments. With advanced techniques like 2D NMR and on-demand precision testing, we deliver the reliable data scientists need to drive discovery and development forward.

Training & Audits
Expert-led GMP/GLP training and independent third-party audits enhance pharmaceutical quality, compliance, and operational excellence. With over 60 training programs and 110 audits completed, these services empower professionals with practical skills and ensure alignment with global regulatory standards—supporting safer, research-driven pharmaceutical development and manufacturing.

Other
We provide advanced lab solutions, FTE-based services, and specialist consulting to assist your study. Under the direction of seasoned Ph.D. scientists, our team guarantees confidentiality, open communication, and dependable outcomes. Help us to meet world standards and confidently advance your research by producing efficient, cost-effective results.