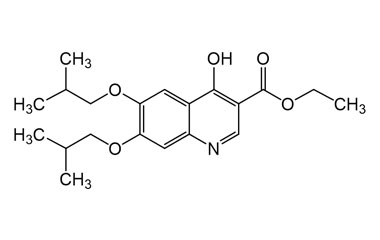
Buquinolate
Impurities
- Recently the U. S. Food and Drug Administration approved buquinolate as a feed additive to aid in preventing coccidiosis due to Eimeria tenella, E. necatrix, E. acervulina, and E. maxima in broiler chickens. Reference standards of Buquinolate API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => Buquinolate
[catalogue_number] => PA 29 59000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 361.44
[form] => C20H27NO5
[cas] => 5486-03-3
[pslug] => 5486-03-3-buquinolate-api-pa2959000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 29 59000
Molecular Formula : C20H27NO5
Molecular Weight : 361.44