alosetron hydrochloride
Miscellaneous compounds
- Alosetron Hydrochloride is a 5-HT3 antagonist used only for the management of severe diarrhoea-predominant irritable bowel syndrome in women. It was patented in 1987 and approved for medical use in 2002. Reference standards of Alosetron Hydrochloride API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
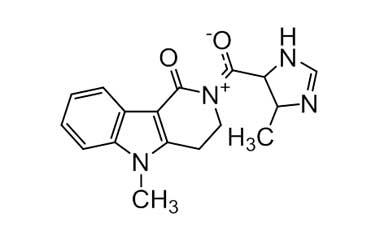
[pname] => 5-Methyl-2-(4-methyl-4,5-dihydro-1H-imidazole-5-carbonyl)-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one
[catalogue_number] => PA 28 22550
[category_ids] => ,84,80,
[chemical_name] =>
[weight] => 310.36
[form] => C17H18N4O2
[cas] => NA
[pslug] => 5-methyl-2-4-methyl-4-5-dihydro-1h-imidazole-5-carbonyl-2-3-4-5-tetrahydro-1h-pyrido-4-3-b-indol-1-one-pa2822550
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
5-Methyl-2-(4-methyl-4,5-dihydro-1H-imidazole-5-carbonyl)-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one
Catalogue No.:PA 28 22550
Molecular Formula : C17H18N4O2
Molecular Weight : 310.36