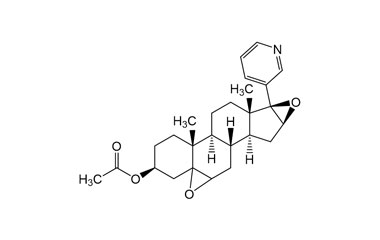
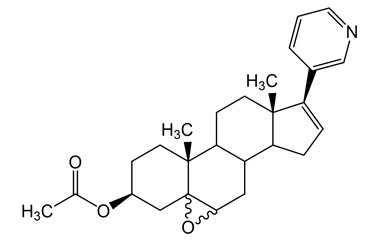
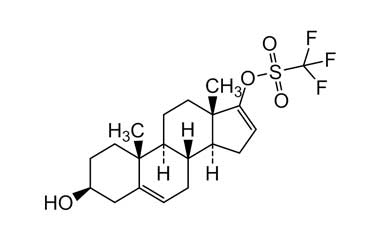
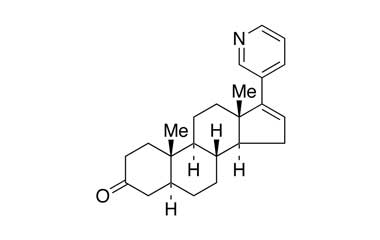
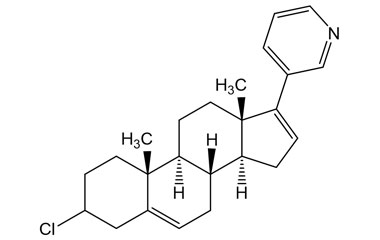
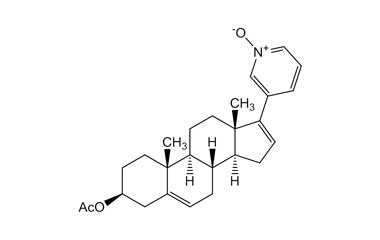
Abiraterone Acetate Unknown Impurity (PAI01004001) is a high-purity pharmaceutical impurity reference standard supplied by Pharmaffiliates for advanced scientific and analytical applications. This impurity material is associated with Abiraterone Acetate and is commonly utilized in analytical method development, impurity profiling, and quality control studies within pharmaceutical research environments.
Designed to support rigorous R&D and regulated laboratory use, this impurity reference enables accurate identification, characterization, and quantification of related process or degradation by-products. It is suitable for method validation, stability studies, and regulatory submission support in pharmaceutical development programs.