Ondansetron- A globally accepted Antiemetic drug

A recent report by Medspace suggests that Acute Gastroenteritis is responsible for some 600,000 hospitalizations and 5000 deaths a year in the United States. Patients who are unable to tolerate fluid replenishment with oral hydration may need to receive intravenous (IV) fluids in the emergency department.
The most commonly used medicine for empiric treatment is Ondansetron. World Health Organization’s (WHO) generates a list of Essential Medicines, which is a list of medications that are considered to be the most effective and safe with regards to meeting the most important needs in a health system and Ondansetron appears on this list.
Ondansetron is a selective 5-HT3 serotonin-receptor antagonist and it is 1 of 4 FDA-approved 5-HT3 serotonin-receptor antagonists used to combat nausea and vomiting.
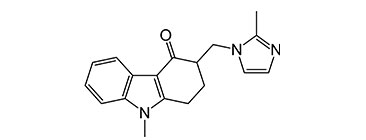
CAS No - 99614-02-5
Ondansetron is hence a widely used antiemetic drug that is considered safe for all age groups with low toxicity and adverse effects. But to obtain a drug product with high purity and good efficacy, Pharmaceutical manufacturers need to check impurities during the development, scale-up and manufacturing processes.
Raw material and excipients play an important role in formulating drug products. The formation of impurities in the drug products is related to various interactions like drug-excipient, excipient-impurity, or impurity-API, excipient-API which leads to compromised quality or performance of the medication.
Henceforth, the impurities need to be checked in both drug material and drug products. Some of the Pharmacopeial and non-Pharmacopeial of Ondansetron which were identified, isolated and characterized are tabulated below:
| Chemical Name | Product Name | Cas No. | Remarks |
|---|---|---|---|
| Ondansetron | Ondansetron - API | 99614-02-5 | API |
| (3RS)-3-(Dimethylamino)-methyl-9-methyl-1,2,3,9-tetrahydro-4Hcarbazol-4-one Hydrochloride | Ondansetron - Impurity A | 119812-29-2 | Pharmacopeial imp of EP |
| 6,6′-Methylene-bis[(3RS)-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one | Ondansetron - Impurity B | 1076198-52-1 | Pharmacopeial imp of EP |
| 9-Methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one | Ondansetron - Impurity C | 27387-31-1 | Pharmacopeial imp of EP |
| 9-Methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one | Ondansetron - Impurity D | 99614-64-9 | Pharmacopeial imp of EP |
| Imidazole | Ondansetron - Impurity E | 288-32-4 | Pharmacopeial imp of EP |
| 2-Methyl-1H-imidazole | Ondansetron - Impurity F | 693-98-1 | Pharmacopeial imp of EP |
| C-Desmethyl Ondansetron | Ondansetron - Impurity G | 99614-03-6 | Pharmacopeial imp of EP |
| N-Demethyl Ondansetron | Ondansetron - Impurity H | 99614-14-9 | Pharmacopeial imp of EP |
| 4-Methylimidazole | 4-Methylimidazole | 822-36-6 | In House impurity |
| 1-(Methoxymethyl)-1H-imidazole | 1-(Methoxymethyl)-1H-imidazole | 20075-26-7 | In House impurity |
| 1-((2-Chlorophenyl)diphenylmethyl)-2-methyl-1H-imidazole | 1-((2-Chlorophenyl)diphenylmethyl)-2-methyl-1H-imidazole | 98751-53-2 | In House impurity |
| 1-((2-Chlorophenyl)diphenylmethyl)-4-methyl-1H-imidazole | 1-((2-Chlorophenyl)diphenylmethyl)-4-methyl-1H-imidazole | NA | In House impurity |
| 4-Hydroxy-3-methyl-9H-carbazol | 4-Hydroxy-3-methyl-9H-carbazol | 150355-51-4 | In House impurity |
| 6-Methyl-9H-carbazol-4-ol | 6-Methyl-9H-carbazol-4-ol | NA | In House impurity |
| 4-Hydroxy-9-methyl Carbazole | 4-Hydroxy-9-methyl Carbazole | 247096-42-0 | In House impurity |
| 9-Methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-1-methylene-2,3-dihydro-1H-carbazol-4(9H)-one | 9-Methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-1-methylene-2,3-dihydro-1H-carbazol-4(9H)-one | NA | In House impurity |
| Ondansetron Resolution Mixture | Ondansetron Resolution Mixture | NA | In House impurity |
| 2,3-Dihydro-1H-carbazol-4(9H)-one | 2,3-Dihydro-1H-carbazol-4(9H)-one | 15128-52-6 | In House impurity |
| 3-Methylene-2,3-dihydro-1H-carbazol-4(9H)-one | 3-Methylene-2,3-dihydro-1H-carbazol-4(9H)-one | NA | In House impurity |
| 1-(Benzofuran-2-yl)butan-1-one | 1-(Benzofuran-2-yl)butan-1-one | 85614-50-2 | In House impurity |
| 1-(Benzofuran-2-yl)butan-1-ol | 1-(Benzofuran-2-yl)butan-1-ol | 1342520-64-2 | In House impurity |
| 2-(1-Methoxybutyl)benzofuran | 2-(1-Methoxybutyl)benzofuran | 1391052-05-3 | In House impurity |
| Methyl 4-hydroxybenzoate | Methyl 4-hydroxybenzoate | 99-76-3 | |
| Methyl 4-hydroxy-3,5-diiodobenzoate | Methyl 4-hydroxy-3,5-diiodobenzoate | 3337-66-4 | In House impurity |
| Methyl 4-(2-(diethylamino)ethoxy)-3,5-diiodobenzoate | Methyl 4-(2-(diethylamino)ethoxy)-3,5-diiodobenzoate | NA | In House impurity |
| 4-(2-(Diethylamino)ethoxy)-3,5-diiodobenzoic Acid | 4-(2-(Diethylamino)ethoxy)-3,5-diiodobenzoic Acid | NA | In House impurity |
| 4-(2-(Diethylamino)ethoxy)-3,5-diiodobenzoyl Chloride | 4-(2-(Diethylamino)ethoxy)-3,5-diiodobenzoyl Chloride | NA | In House impurity |
| 1,1’-(Methylenedi-4,1-phenylene)bishydrazine Dihydrochloride | 1,1’-(Methylenedi-4,1-phenylene)bishydrazine Dihydrochloride | 100829-65-0 | In House impurity |
| 7,7'-Methylenebis(1,2,3,9-tetrahydro-4H-carbazol-4-one) | 7,7'-Methylenebis(1,2,3,9-tetrahydro-4H-carbazol-4-one) | NA | In House impurity |
| 7,7'-methylenebis(9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one) | 7,7'-methylenebis(9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one) | NA | In House impurity |
| 7,7'-Methylenebis(9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one) | 7,7'-Methylenebis(9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one) | NA | In House impurity |
During the analysis of any drug appropriate methods, procedures, and practices have to be designed and applied to ensure that the result will meet "fitness-for-purpose" requirements. Here the requirement of a reference standard comes into play.
The application of reference standards in ascertaining the quality of drugs and pharmaceuticals which is now well established and followed by a wide range of international, national and professional organizations. The purpose of having reference standards is to achieve the accuracy and reproducibility of the analytical results required by pharmacopeial testing and pharmaceutical control in general.
A reliable and trusted partner can provide a traceable reference standard along with a Certificate of Analysis (CoAs) which includes all the supporting documents required to fulfill the regulatory requirements.
The reference standards of Ondansetron impurities can be obtained from globally recognized research organization Pharmaffiliates Analytics and Synthetics Pvt. Ltd.





