Temozolomide- A miracle for glioblastoma (GBM) patients

Studies indicate, that nearly 5–8/100,000 population suffer from Malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) which occurs more frequently than other types of primary central nervous system tumors.
Even after many aggressive treatments like surgery, radiation, and chemotherapy, the survival chance is less than one year.
Temozolomide,(TMZ) a new drug, has shown promise in treating malignant gliomas and other difficult-to-treat tumors.
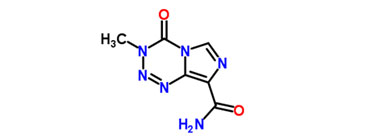
Temozolomide is a leading new class of chemotherapeutic agents that enter the cerebrospinal fluid and do not require hepatic metabolism for activation. Chemically, it is an imidazotetrazine second-generation alkylating agent.
Temozolomide has been in news recently for a great reason. Scientists from Akay Lab biomedical research team at the University of Houston have designed a microfluidic brain cancer chip which allows multiple-simultaneous drug administration, and a massive parallel testing of drug response for patients with glioblastoma (GBM), the most common malignant brain tumor.
Scientists investigated the effect of the combination of Temozolomide and a nuclear factor-κB inhibitor on tumor growth.
Temozolomide or TMZ works by breaking the DNA double-strand, thus causing cell cycle arrest and ultimately cell death. However, due to its short half-life, TMZ is administered at a high dose, and due to prolonged systemic administration results in a series of side effects. To improve the efficacy of TMZ and reduce the side effects of chemotherapy, systemic TMZ administration using a biodegradable carrier such as nanoparticles is also used.
Thus, the medical world uses combined strategies for the development of new therapeutic agents to prolong survival and improve the quality of life of GBM patients.
Drug development and manufacturing of Temozolomide involves the formation of several impurities in the form of degradants, intermediates, and metabolites affecting the efficacy, safety, and purity of the final products.
Identification and isolation of these impurities is a major challenge for the pharmaceutical companies and it is gaining a critical review from the regulatory authorities as well.
Some of the known and unknown impurities of Temozolomide and its Stable Isotopes are listed below :
| Type of impurity | Chemical Name | Product Name | Cas No. |
|---|---|---|---|
| API | Temozolomide | Temozolomide | 85622-93-1 |
| Known impurity of BP/EP | 4-Amino-1H-imidazole-5-carboxamide | Temozolomide - Impurity A | 360-97-4 |
| Unknown impurity | Temozolomide Acid | Temozolomide | 113942-30-6 |
| Unknown impurity | 4-Amino-1H-imidazole-5-carbonitrile | 4-Amino-1H-imidazole-5-carbonitrile | 03-11-98 |
| Known impurity of BP/EP | 4-Diazo-4H-imidazole-5-carboxamide | Temozolomide - Impurity D | 7008-85-7 |
| Unknown impurity | Monomethyl Triaizeno Imidazole Carboxamide | Monomethyl Triaizeno Imidazole Carboxamide | 3413-72-7 |
| Known impurity of BP/EP | 2-Azahypoxanthine | Temozolomide - Impurity E | 4656-86-4 |
| Unknown impurity | Cyanotemozolomide | Cyanotemozolomide | 287964-59-4 |
| Unknown impurity | 5-Amino-1-(N-methylcarbamoyl)imidazole-4-carboxamide | 5-Amino-1-(N-methylcarbamoyl)imidazole-4- | 188612-53-5 |
| Unknown impurity | 4-Nitrophenol | 4-Nitrophenol | 100-02-7 |
| Unknown impurity | (1H-Imidazole-4-carboxamide,5-amino-2-[2-[4-(aminocarbonyl)-1H-imidazol-5-yl]diazenyl]-) | (1H-Imidazole-4-carboxamide,5-amino-2-[2-[4-(aminocarbonyl)-1H-imidazol-5-yl]diazenyl]-) | 87614-68-4 |
| Known impurity of BP/EP | Temozolomide Cyano Impurity | Temozolomide - Impurity C | 114601-31-9 |
| Unknown impurity | 5-Amino-1H-imidazole-4-carboxamide Hydrate | 5-Amino-1H-imidazole-4-carboxamide Hydrate | 64236-33-5 |
| Unknown impurity | N,N-Diphenylcarbamyl Chloride | N,N-Diphenylcarbamyl Chloride | 83-01-2 |
| Stable isotope | 3-Methyl-d3-1,1-diphenylurea | 3-Methyl-d3-1,1-diphenylurea | 208107-10-2 |
| Stable isotope | 5-Aminoimidazole-4-carboxamide-13C2,15N Hydrochloride Salt | 5-Aminoimidazole-4-carboxamide-13C2,15N Hydrochloride Salt | 1246816-45-4 |
| Stable isotope | MTIC-d3 | MTIC-d3 | 1185242-69-6 |
| Stable isotope | Temozolomide-d3 | Temozolomide-d3 | 208107-14-6 |
| Known impurity of BP/EP | 5-Amino-1H-imidazole-4-carboxamide Hydrochloride | Temozolomide - Impurity A (Hydrochloride Salt) | 72-40-2 |
| Unknown impurity | 3-Amino-3-imino-2-(2-phenyldiazenyl)propanamide Hydrochloride | 3-Amino-3-imino-2-(2-phenyldiazenyl)propanamide | 6285-64-9 |
| Unknown impurity | 3-Amino-2-(formylamino)-3-iminopropanamide | 3-Amino-2-(formylamino)-3-iminopropanamide | 100191-43-3 |
These are some of the potential impurities of Temozolomide which need to be taken care of in the final drug product. For this, there is a requirement of highly pure reference material against which the sample can be tested. Reference materials are an integral component of the procedures of the private or public control specification.
Therefore, choosing and procuring the best quality and high purity Pharmaceutical Reference Standards and other key reagents like metabolites, intermediates, stable isotopes and chiral standards of a drug is critical and finding a reliable source and supplier of Reference standards is an uphill task for the organizations.
The Reference standards provider must also provide the Source, Lot number, Expiration, COA (evidence of identity and purity) with each impurity standard as per the global regulatory authorities.
Pharmaffiliates Analytics and Synthetics Pvt. Ltd. is a world-class manufacturer, supplier and exporter of Reference Standards which fulfills all the above-mentioned requirements along with providing the best prices and high quality.
Pharmaffiliates is a global name that operates in more than 60 countries worldwide







