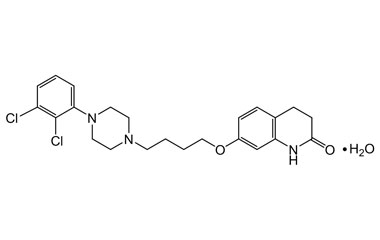
Aripiprazole Monohydrate and its Impurities
Aripiprazole Monohydrate was approved by FDA (Abilify trade name) for the treatment of schizophrenia; manic and mixed episodes associated with bipolar I disorder; major depressive disorder; irritability associated with autistic disorder; Tourette's disorder and agitation associated with schizophrenia or bipolar mania. Reference standards of Aripiprazole Monohydrate API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.