cabazitaxel
Antibody Drug Conjugate - Cabazitaxel was developed by Sanofi-Aventis and was approved by the U.S. FDA for the treatment of hormone-refractory prostate cancer on June 17, 2010. It is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.. Reference standards of Cabazitaxel API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below

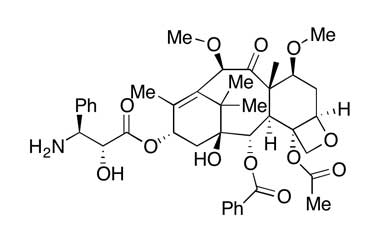
De-boc Cabazitaxel
Catalogue No.:PA 03 01510
CAS :
1638286-64-2
Molecular Formula : C40H49NO12
Molecular Weight : 735.82







