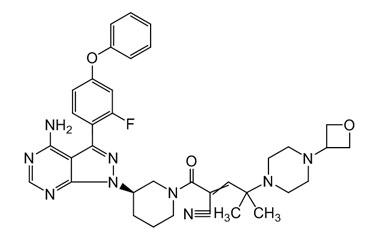
Rilzabrutinib
Metabolites
- Rilzabrutinib for the Treatment of Chronic Spontaneous Urticaria in Patients Who Remain Symptomatic Despite the Use of H1 Antihistamine and Who Are naïve to Omalizumab (RILECSU). Reference standards of Rilzabrutinib API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => Rilzabrutinib
[catalogue_number] => PA 18 1270000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 665.77
[form] => C36H40FN9O3
[cas] => 1575596-29-0
[pslug] => 1575596-29-0-rilzabrutinib-api-pa181270000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 18 1270000
Molecular Formula : C36H40FN9O3
Molecular Weight : 665.77
stdClass Object
(
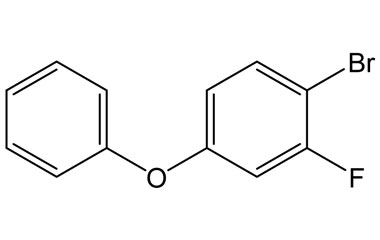
[pname] => 1-Bromo-2-fluoro-4-phenoxybenzene
[catalogue_number] => PA 18 1271000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 267.1
[form] => C12H8BrFO
[cas] => 1138557-58-0
[pslug] => 1138557-58-0-1-bromo-2-fluoro-4-phenoxybenzene-pa181271000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
1-Bromo-2-fluoro-4-phenoxybenzene
stdClass Object
(
[pname] => 1-Bromo-2-fluoro-4-phenoxybenzene
[catalogue_number] => PA 18 1271000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 267.1
[form] => C12H8BrFO
[cas] => 1138557-58-0
[pslug] => 1138557-58-0-1-bromo-2-fluoro-4-phenoxybenzene-pa181271000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)