cabazitaxel
Miscellaneous compounds
- Cabazitaxel was developed by Sanofi-Aventis and was approved by the U.S. FDA for the treatment of hormone-refractory prostate cancer on June 17, 2010. It is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.. Reference standards of Cabazitaxel API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below
stdClass Object
(
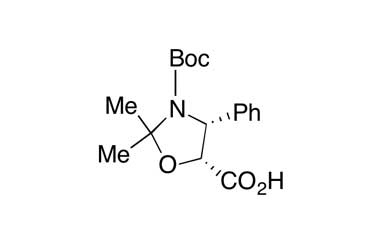
[pname] => (4R,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic Acid
[catalogue_number] => PA 03 01620
[category_ids] => ,84,
[chemical_name] =>
[weight] => 321.37
[form] => C17H23NO5
[cas] => NA
[pslug] => 4r-5r-3-tert-butoxycarbonyl-2-2-dimethyl-4-phenyloxazolidine-5-carboxylic-acid-pa0301620
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(4R,5R)-3-(tert-Butoxycarbonyl)-2,2-dimethyl-4-phenyloxazolidine-5-carboxylic Acid
Catalogue No.:PA 03 01620
Molecular Formula : C17H23NO5
Molecular Weight : 321.37