| Catalogue number: | PA 02 0791011 |
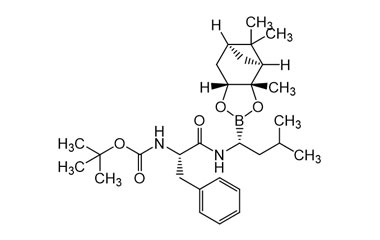
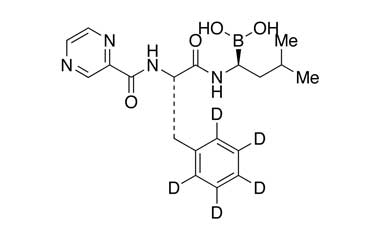
| Chemical name: | N-[(1S)-2-[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methano-1,3,2-benzodioxaborol-2-yl]-3-methylbutyl]amino]-2-oxo-1-(phenylmethyl)ethyl]-carbamic acid 1,1-dimethylethyl ester |
CAS Number: |
1187479-72-6 |
| Category: | impurities,metabolites,pharmaceutical standards,intermediates,Fine Chemicals |
| Synonyms: | Tert-Butyl ((S)-1-(((R)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate; |
| Molecular form: | C29H45BN2O5 |
| Appearance: | NA |
| Mol. Weight: | 512.5 |
| Storage: | 2-8°C Refrigerator |
| Shipping Conditions: | Ambient |
| Applications: | N-[(1S)-2-[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methano-1,3,2-benzodioxaborol-2-yl]-3-methylbutyl]amino]-2-oxo-1-(phenylmethyl)ethyl]-Carbamic Acid 1,1-Dimethylethyl Ester is an intermediate in the synthesis of bortezomib (B675700).Bortezomib is the first proteasome inhibitor to be approved by the US FDA for multiple myeloma, a blood cancer. |
N-[(1S)-2-[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methano-1,3,2-benzodioxaborol-2-yl]-3-methylbutyl]amino]-2-oxo-1-(phenylmethyl)ethyl]-carbamic acid 1,1-dimethylethyl ester
In House ImpurityEnquire for N-[(1S)-2-[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methano-1,3,2-benzodioxaborol-2-yl]-3-methylbutyl]amino]-2-oxo-1-(phenylmethyl)ethyl]-carbamic acid 1,1-dimethylethyl ester
Related Products
Search by Keywords – Buy 1187479-72-6 | Purchase 1187479-72-6 | Order 1187479-72-6 | Enquire 1187479-72-6 | Price of 1187479-72-6 | 1187479-72-6 Cost | 1187479-72-6 Supplier | 1187479-72-6 Distributor | 1187479-72-6 Manufacturer | 1187479-72-6 Exporter