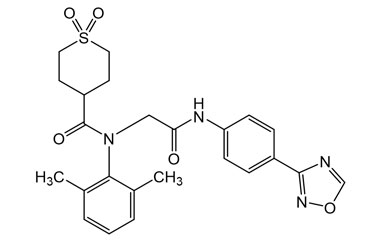
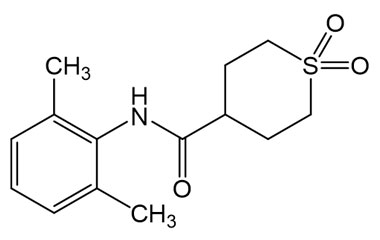
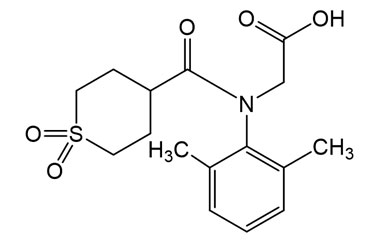
Amenamevir and its Impurities
Amenamevir trade name Amenalief is an antiviral drug . it has been used in trials studying the treatment of Herpes Zoster, Herpes Simplex, Herpes Genitalis. Reference standards of Amenamevir API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.