amoxapine and its Impurities
Amoxapine first received marketing approval in the United States in 1992. It is used in the treatment of major depressive disorder. Reference standards of Amoxapine API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

![4-(2-Chlorodibenzo[b,f][1,4]oxazepin-11-yl)-N-[2-( PA 28 0721005](https://www.pharmaffiliates.com/pimages/PA280721005.jpg)
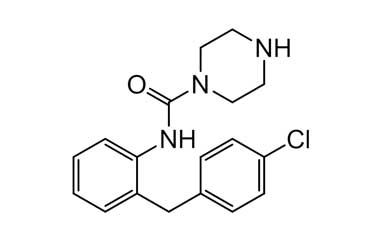
4-(2-Chlorodibenzo[b,f][1,4]oxazepin-11-yl)-N-[2-(4-chlorophenoxy)phenyl]piperazine-1-carboxamide
4-(2-Chlorodibenzo[b,f][1,4]oxazepin-11-yl)-N-[2-(4-chlorophenoxy)phenyl]piperazine-1-carboxamide
Catalogue No.:PA 28 0721005
CAS :
2835304-63-5
Molecular Formula : C30H24Cl2N4O3
Molecular Weight : 559.45

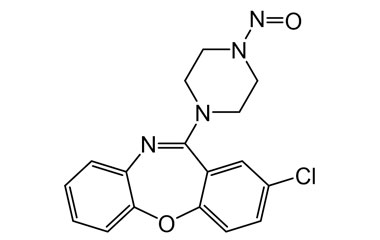
![N-2-Chlorobenz-[b,f][1,4]oxazepine-11-yl Amoxapine PA 28 72520](https://www.pharmaffiliates.com/pimages/PA2872520.jpg)
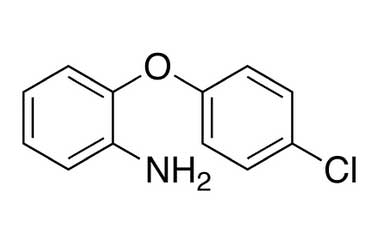
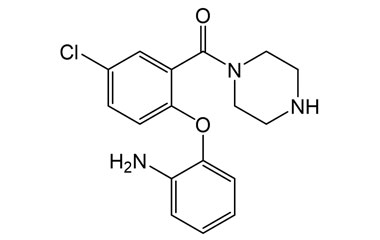
![Ethyl 4-{[o-(p-chlorophenoxy)phenyl]-carbamoyl}-1- PA 28 72540](https://www.pharmaffiliates.com/pimages/PA2872540.jpg)
![2-Chloro-10,11-dihydro-11-oxo-dibenzo[b,f][1,4]oxa PA 28 72550](https://www.pharmaffiliates.com/pimages/PA2872550.jpg)
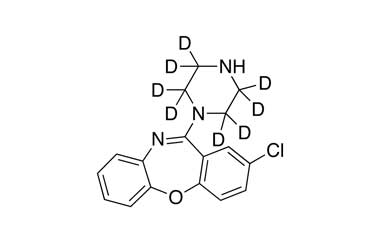
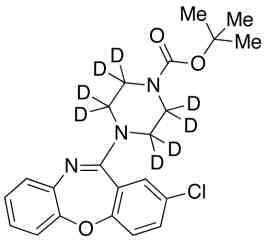
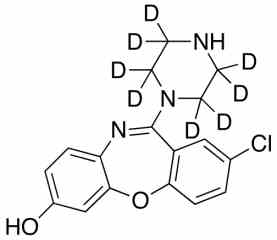




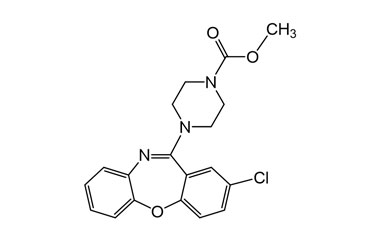
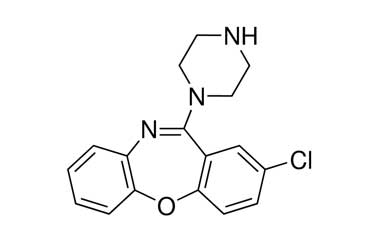
![3-Chloro-11-(piperazin-1-yl)dibenzo[b,f][1,4]oxaze PA 28 0721001](https://www.pharmaffiliates.com/pimages/PA280721001.jpg)
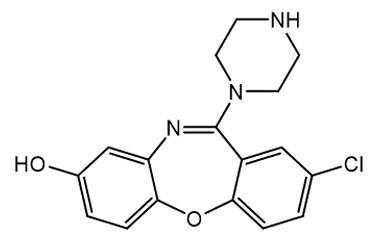
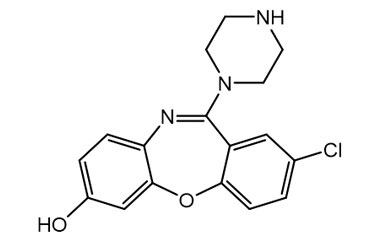
![Ethyl [2-(4-Chlorophenoxy)phenyl]carbamate PA 28 0721004](https://www.pharmaffiliates.com/pimages/PA280721004.jpg)