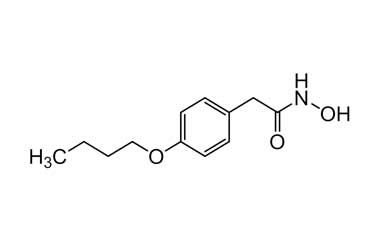
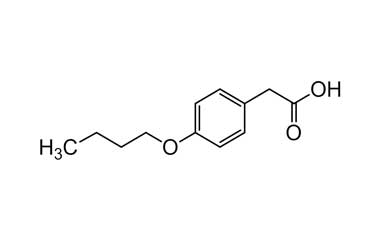
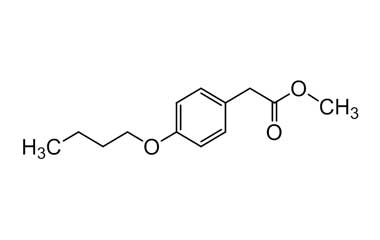
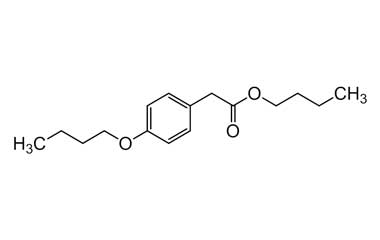
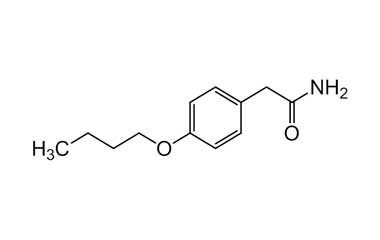
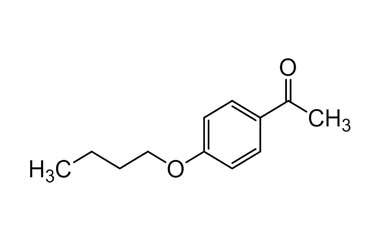
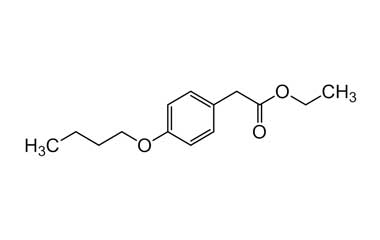
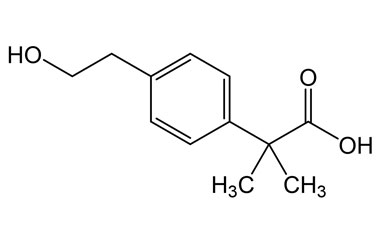
bufexamac and its Impurities
Bufexamac was withdrawn in Europe because of allergic reactions. It is a drug used as an anti-inflammatory agent on the skin, as well as rectally. Common brand names include Paraderm and Parfenac.. Reference standards of Bufexamac API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below