ceftriaxone disodium salt hemi(heptahydrate) and its Impurities
Ceftriaxone Disodium Salt Hemi(Heptahydrate) and other third-generation antibiotics are used to treat organisms that tend to be resistant to many other antibiotics. Reference standards of Ceftriaxone Disodium Salt Hemi(Heptahydrate) API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below

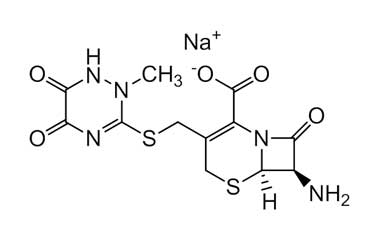
Ceftriaxone Sodium - Impurity E (Sodium Salt)
(6R,7R)-7-Amino-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-1,2,4-triazin-3-yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid Sodium Salt
Catalogue No.:PA 03 97520
CAS :
2227256-62-2
Molecular Formula : C12H12N5NaO5S2
Molecular Weight : 393.37

![(E)-S-Benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl) PA 03 97510](https://www.pharmaffiliates.com/pimages/PA0397510.jpg)





