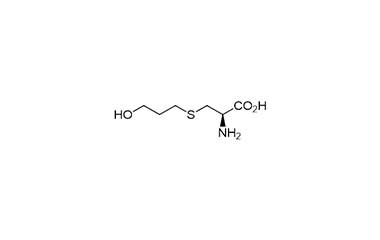
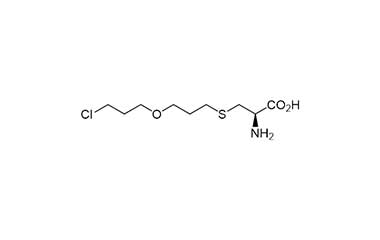
Fudosteine and its Impurities
Fudosteine is a derivative of cysteine developed and approved in Japan for the treatment of such diseases as bronchial asthma, chronic bronchitis, pulmonary emphysema, bronchiectasis, pulmonary tuberculosis, pneumoconiosis, atypical mycobacterial disease, and diffuse panbronchiolitis. Reference standards of Fudosteine API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.