rifamycin sodium and its Impurities
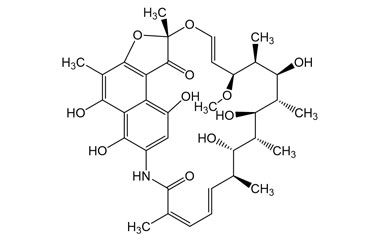
It is the hydroquinone form Rifamycin S, formed by the reduction of the active form Rifamycin S. Rifamycin SV became the first member of Rifamycin class to enter clinical use as an intravenous antibiotic. Reference standards of Rifamycin Sodium API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

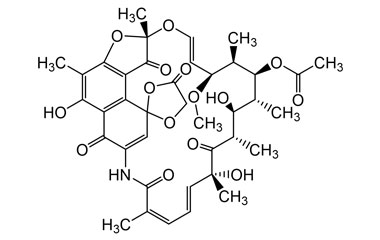
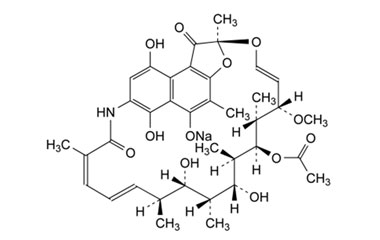
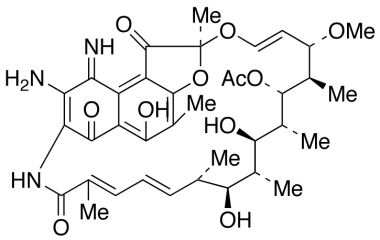
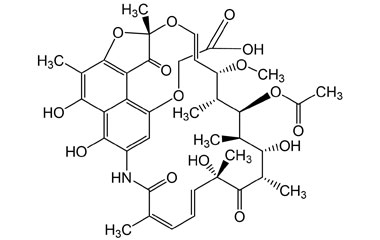
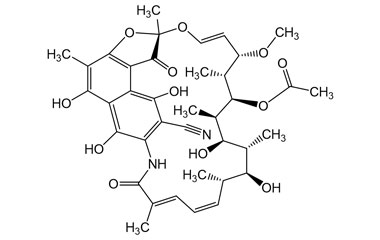
21-Acetate, Spiro[1,3-dioxolane-2,9′(6′H)-[2,7](epoxypentadeca[1,11,13]trienimino)naphtho[2,1-b]furan]-1′,4,6′,11′,17′(2′H)-pentone, 5′,16′,19′,21′-tetrahydroxy-23′-methoxy-2′,4′,12′,16′,18′,20′,22′-heptamethyl
21-Acetate, Spiro[1,3-dioxolane-2,9′(6′H)-[2,7](epoxypentadeca[1,11,13]trienimino)naphtho[2,1-b]furan]-1′,4,6′,11′,17′(2′H)-pentone, 5′,16′,19′,21′-tetrahydroxy-23′-methoxy-2′,4′,12′,16′,18′,20′,22′-heptamethyl
Catalogue No.:PA 18 0581001
CAS :
17778-33-5
Molecular Formula : C39H45NO15
Molecular Weight : 767.78