cobimetinib
Intermediates
- It has been approved in Switzerland and the US, in combination with vemurafenib for the treatment of patients with unresectable or metastatic BRAF V600 mutation-positive melanoma.. Reference standards of Cobimetinib API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below
stdClass Object
(
[pname] => Cobimetinib
[catalogue_number] => PA 56 64000
[category_ids] => ,80,71,78,70,82,121,
[chemical_name] =>
[weight] => 531.31
[form] => C21H21F3IN3O2
[cas] => 934660-93-2
[pslug] => 934660-93-2-cobimetinib-api-pa5664000
[latest_product] => 0
[linkproducts] => 1
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
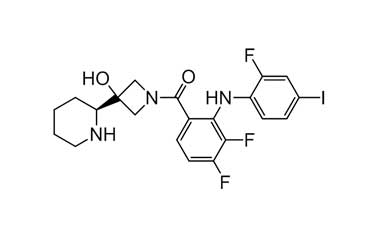
Catalogue No.:PA 56 64000
Molecular Formula : C21H21F3IN3O2
Molecular Weight : 531.31
stdClass Object
(
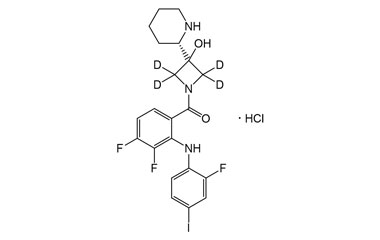
[pname] => Cobimetinib-D4 HCl
[catalogue_number] => PA STI 089945
[category_ids] => ,80,71,78,70,82,121,98,
[chemical_name] =>
[weight] => 571.8
[form] => C21H18D4ClF3IN3O2
[cas] => NA
[pslug] => cobimetinib-d4-hcl-pasti089945
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)