amprenavir and its Impurities
Amprenavir was patented in 1992 and approved for medical use in 1999.It inhibitor used to treat HIV infection. Reference standards of Amprenavir API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

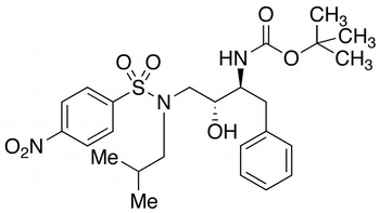
[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-nitro-phenyl)sulfonyl)]amino]propyl]-carbamic Acid tert-Butyl Ester
[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-nitro-phenyl)sulfonyl)]amino]propyl]-carbamic Acid tert-Butyl Ester
Catalogue No.:PA STI 011510
CAS :
191226-98-9
Molecular Formula : C25H35N3O7S
Molecular Weight : 521.63

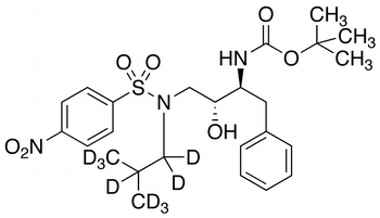
[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl-d9-[(4-nitrophenyl)sulfonyl]amino] propyl]carbamic Acid tert-Butyl Ester
[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl-d9-[(4-nitrophenyl)sulfonyl]amino] propyl]carbamic Acid tert-Butyl Ester
Catalogue No.:PA STI 011520
CAS :
NA
Molecular Formula : C25H26D9N3O7S
Molecular Weight : 530.68


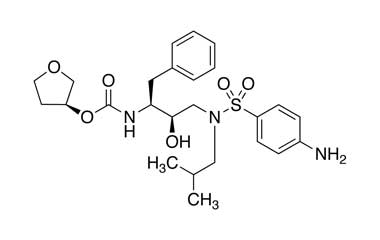
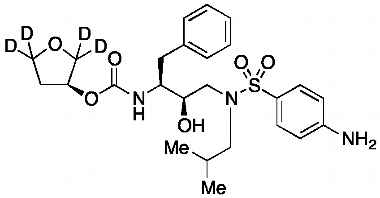
[(1S,2R)-3-[[(4-Nitrophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-phenylmethyl)propyl]carbamic Acid, (3S)-Tetrahydro-3-furanyl Ester
[(1S,2R)-3-[[(4-Nitrophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-phenylmethyl)propyl]carbamic Acid, (3S)-Tetrahydro-3-furanyl Ester
Catalogue No.:PA 28 93510
CAS :
160231-69-6
Molecular Formula : C25H33N3O8S
Molecular Weight : 535.61