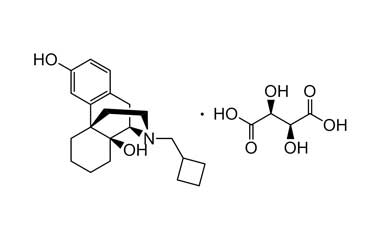
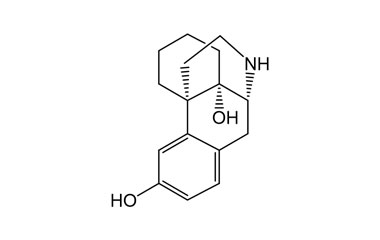
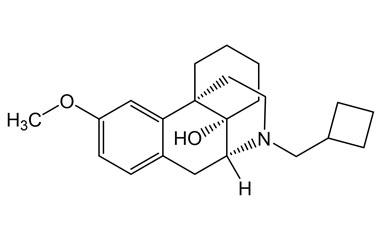
butorphanol tartrate and its Impurities
The most common indication for Butorphanol Tartrate is management of migraine using the intranasal spray formulation. It is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers.. Reference standards of Butorphanol Tartrate API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below