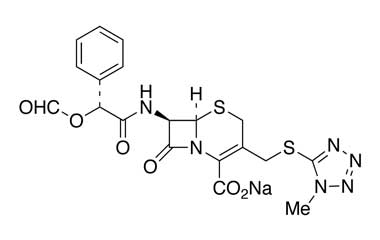
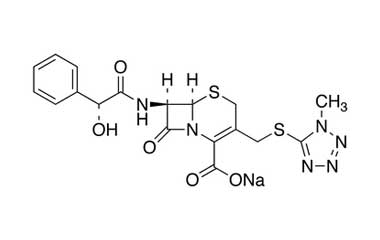
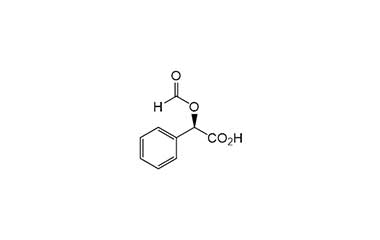
cefamandole nafate and its Impurities
The clinically used form of cefamandole is the formate ester cefamandole nafate, a prodrug which is administered parenterally. It is no longer available in the United States. Cefamandole Nafate is a second-generation broad-spectrum cephalosporin antibiotic. Reference standards of Cefamandole Nafate API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below