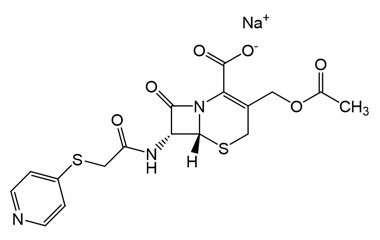
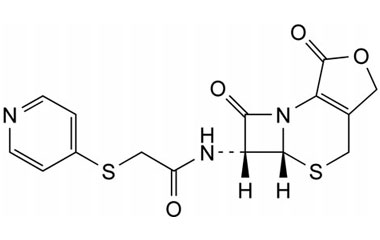
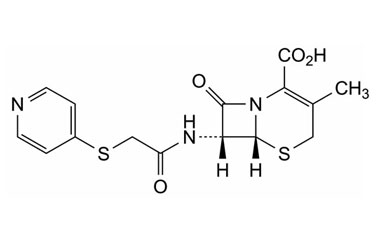
cephapirin sodium and its Impurities
Cephapirin Sodium is marketed under the trade name Cefadyl. It is used for the treatment of mastitis in cows. Reference standards of Cephapirin Sodium API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.