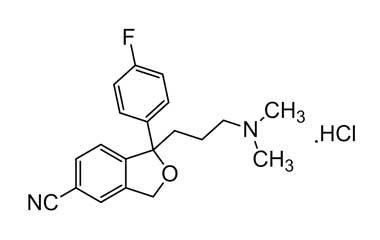
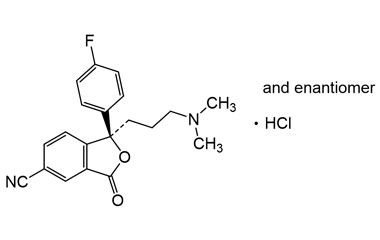
citalopram hydrochloride and its Impurities
Citalopram Hydrochloride was approved for medical use in the United States in 1998. It is sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia.. Reference standards of Citalopram Hydrochloride API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below