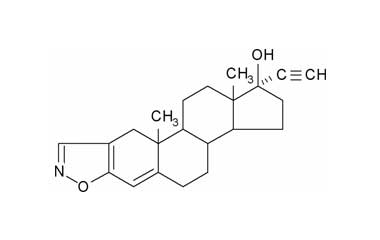
danazol and its Impurities
Danazol is used primarily in the treatment of endometriosis. Danazol, sold as Danocrine and other brand names, is a medication used in the treatment of endometriosis, fibrocystic breast disease, hereditary angioedema and other conditions.. Reference standards of Danazol API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below