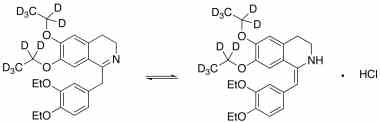
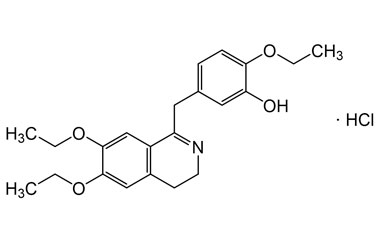
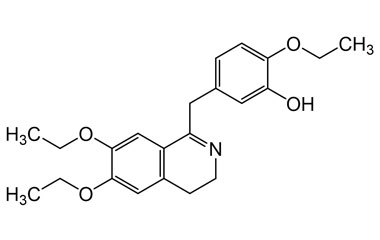
drotaverine and its Impurities
Drotaverine is used to enhance cervical dilation during childbirth. It is available in Asian and Central and Eastern European countries under several brand names. Reference standards of Drotaverine API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.