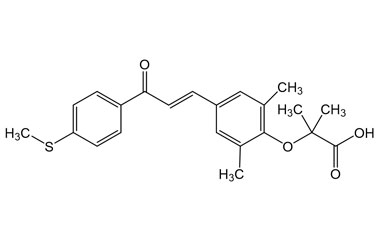
Elafibranor and its Impurities
Elafibranor brand name - Iqirvo is a novel oral medication approved for the treatment of primary biliary cholangitis (PBC), a chronic autoimmune liver disease. Reference standards of Elafibranor API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

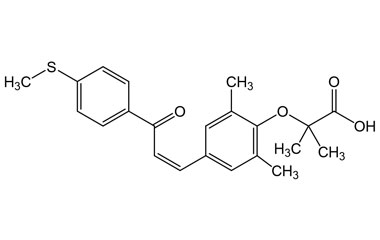
(Z)-2-(2,6-Dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
(Z)-2-(2,6-Dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
Catalogue No.:PA 05 2361000
CAS :
3046217-14-2
Molecular Formula : C22H24O4S
Molecular Weight : 384.49