Epoprostenol Sodium and its Impurities
Epoprostenol Sodium injection is used to treat the symptoms of primary pulmonary hypertension and pulmonary hypertension in patients. Reference standards of Epoprostenol Sodium API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

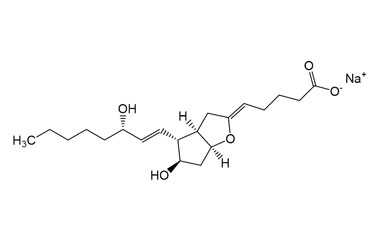
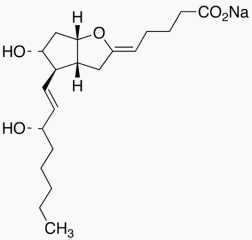
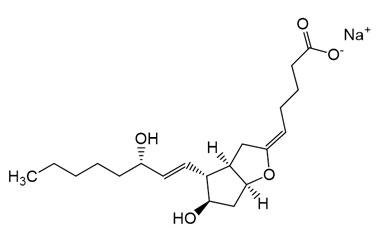
Sodium (Z)-5-((3aR,4R,5R,6aS)-5-hydroxy-4-((S,E)-3-hydroxyoct-1-en-1-yl)hexahydro-2H-cyclopenta[b]furan-2-ylidene)pentanoate
Sodium (Z)-5-((3aR,4R,5R,6aS)-5-hydroxy-4-((S,E)-3-hydroxyoct-1-en-1-yl)hexahydro-2H-cyclopenta[b]furan-2-ylidene)pentanoate
Catalogue No.:PA 32 0281001
CAS :
NA
Molecular Formula : C20H31NaO5
Molecular Weight : 374.45