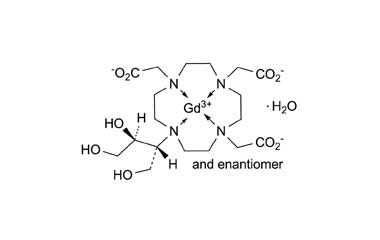
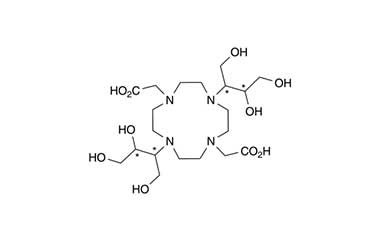
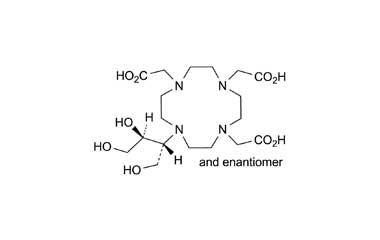
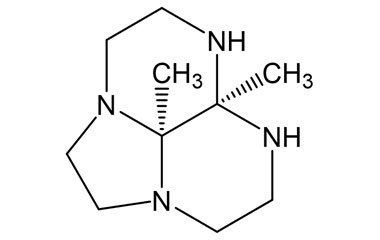
Gadobutrol Monohydrate and its Impurities
Gadobutrol Monohydrate is a medicinal product used in diagnostic magnetic resonance imaging (MRI) in adults and children. It provides contrast enhancement during cranial, spinal, breast, or other investigations. Reference standards of Gadobutrol Monohydrate API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.