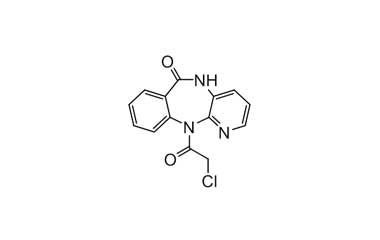
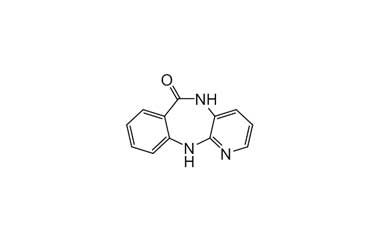
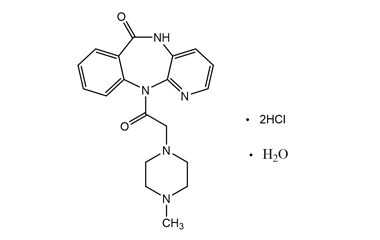
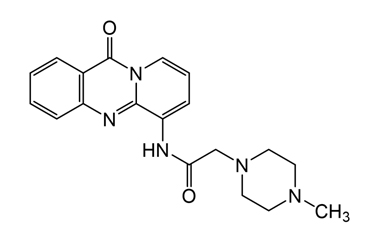
Pirenzepine dihydrochloride monohydrate and its Impurities
Pirenzepine dihydrochloride monohydrate is used in the treatment of peptic ulcers, as it reduces gastric acid secretion and reduces muscle spasm. Reference standards of Pirenzepine Dihydrochloride Monohydrate API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.