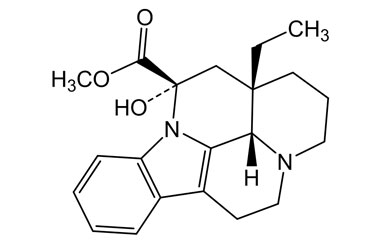
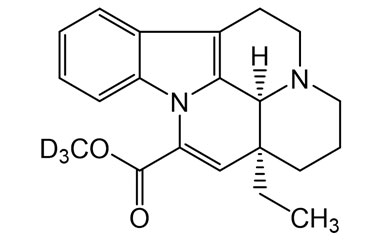
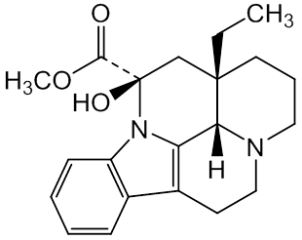
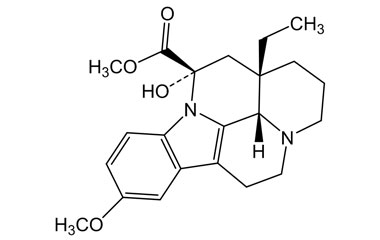
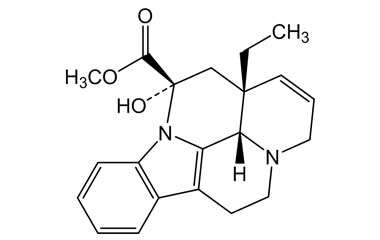
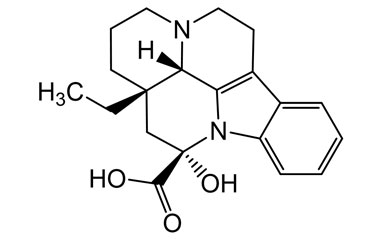
vincamine and its Impurities
Vincamine is permitted to be sold as a dietary supplement when labeled for use in adults for six months or less. It is sold in Europe as a prescription medicine for the treatment of primary degenerative and vascular dementia.Reference standards of Vincamine API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.