amoxapine
Nitrosamine Standards
- Amoxapine first received marketing approval in the United States in 1992. It is used in the treatment of major depressive disorder. Reference standards of Amoxapine API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
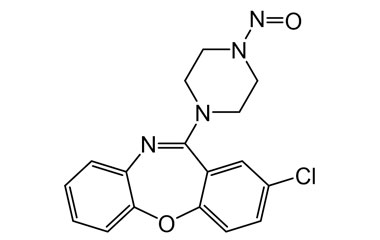
[pname] => N-Nitroso-Amoxapine
[catalogue_number] => PA 28 0721006
[category_ids] => ,70,78,82,162,
[chemical_name] =>
[weight] => 342.78
[form] => C17H15ClN4O2
[cas] => NA
[pslug] => n-nitroso-amoxapine-pa280721006
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 28 0721006
Molecular Formula : C17H15ClN4O2
Molecular Weight : 342.78