bromfenac sodium
Amines
- The FDA and European approvals for Bromfenac Sodium are for use one day before and two weeks following cataract surgery for the treatment of ocular inflammation and pain. It is a nonsteroidal anti-inflammatory drug.. Reference standards of Bromfenac Sodium API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below
stdClass Object
(
[pname] => Bromfenac Sodium
[catalogue_number] => PA 02 47000
[category_ids] => ,79,80,78,70,82,
[chemical_name] =>
[weight] => 356.15
[form] => C15H11BrNNaO3
[cas] => 91714-93-1
[pslug] => 91714-93-1-bromfenac-sodium-api-pa0247000
[latest_product] => 0
[linkproducts] => 1
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
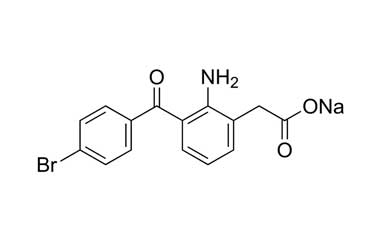
Catalogue No.:PA 02 47000
Molecular Formula : C15H11BrNNaO3
Molecular Weight : 356.15
stdClass Object
(
[pname] => 2-Amino-3-(4-bromobenzoyl)benzoic Acid
[catalogue_number] => PA 02 47510
[category_ids] => ,79,80,76,75,78,70,82,
[chemical_name] =>
[weight] => 320.14
[form] => C14H10BrNO3
[cas] => 241496-82-2
[pslug] => 241496-82-2-2-amino-3-4-bromobenzoyl-benzoic-acid-pa0247510
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
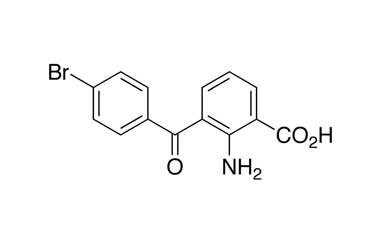
2-Amino-3-(4-bromobenzoyl)benzoic Acid
Catalogue No.:PA 02 47510
Molecular Formula : C14H10BrNO3
Molecular Weight : 320.14