lumiracoxib
Fine chemicals
- Lumiracoxib is a COX-2 selective non-steroidal anti-inflammatory drug . On August 11, 2007, Australia's Therapeutic Goods Administration cancelled the registration of lumiracoxib in Australia due to concerns that it may cause liver failure.Reference standards of Lumiracoxib API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
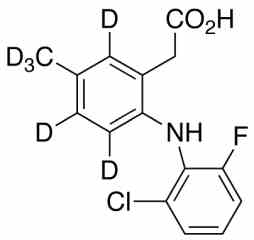
[pname] => Lumiracoxib-d6
[catalogue_number] => PA STI 057310
[category_ids] => ,80,98,78,70,82,
[chemical_name] =>
[weight] => 299.76
[form] => C15H7D6ClFNO2
[cas] => 1225453-72-4
[pslug] => 1225453-72-4-lumiracoxib-d6-pasti057310
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA STI 057310
Molecular Formula : C15H7D6ClFNO2
Molecular Weight : 299.76