vapiprost hydrochloride
Fine chemicals - vapiprost hydrochloride events and occlusive vascular disease. Phase III trials were underway in Japan for the treatment of deep vein thrombosis. Reference standards of Vapiprost Hydrochloride API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

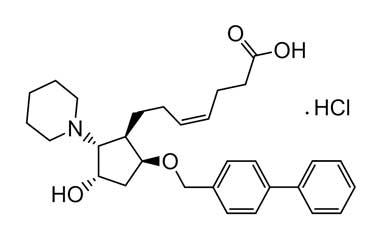
Vapiprost Hydrochloride
Catalogue No.:PA 22 16000
CAS :
87248-13-3
Molecular Formula : C30H40ClNO4
Molecular Weight : 514.10







