Elafibranor
Impurities
- Elafibranor brand name - Iqirvo is a novel oral medication approved for the treatment of primary biliary cholangitis (PBC), a chronic autoimmune liver disease. Reference standards of Elafibranor API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
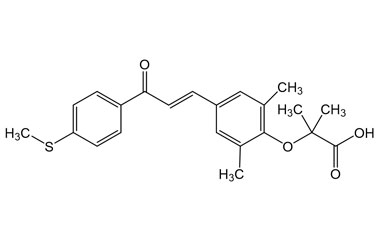
[pname] => Elafibranor
[catalogue_number] => PA 05 2360000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 384.49
[form] => C22H24O4S
[cas] => 923978-27-2
[pslug] => 923978-27-2-elafibranor-pa052360000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 05 2360000
Molecular Formula : C22H24O4S
Molecular Weight : 384.49
stdClass Object
(
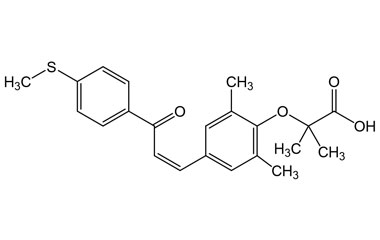
[pname] => (Z)-2-(2,6-Dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
[catalogue_number] => PA 05 2361000
[category_ids] => ,75,76,78,70,82,
[chemical_name] =>
[weight] => 384.49
[form] => C22H24O4S
[cas] => 3046217-14-2
[pslug] => 3046217-14-2z22-6-dimethyl-434methylthio-phenyl3-oxoprop-1-en-1-yl-phenoxy2-methylpropanoic-acid-pa052361000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(Z)-2-(2,6-Dimethyl-4-(3-(4-(methylthio)phenyl)-3-oxoprop-1-en-1-yl)phenoxy)-2-methylpropanoic Acid
Catalogue No.:PA 05 2361000
Molecular Formula : C22H24O4S
Molecular Weight : 384.49