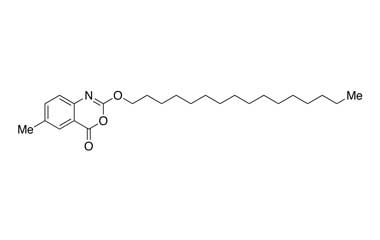
cetilistat
Intermediates
- Cetilistat has completed Phase 1 and 2 trials in the West and is currently in Phase 3 trials in Japan where it is partnered with Takeda. It is a drug designed to treat obesity. It acts in the same way as the older drug orlistat (Xenical) by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Reference standards of Cetilistat API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below
stdClass Object
(
[pname] => Cetilistat
[catalogue_number] => PA 56 25000
[category_ids] => ,80,71,81,78,70,82,
[chemical_name] =>
[weight] => 401.58
[form] => C25H39NO3
[cas] => 282526-98-1
[pslug] => 282526-98-1-cetilistat-api-pa5625000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 56 25000
Molecular Formula : C25H39NO3
Molecular Weight : 401.58