Bictegravir Sodium
Metabolites - Bictegravir Sodium is used a in fixed dose combination with tenofovir alafenamide and emtricitabine for the treatment of HIV-1 infection. Reference standards of Bictegravir Sodium API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

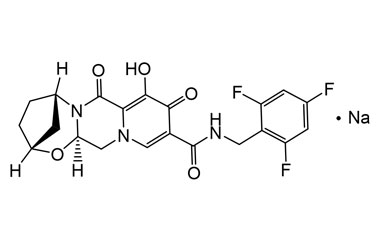
Bictegravir Sodium
Catalogue No.:PA 02 2460000
CAS :
1807988-02-8
Molecular Formula : C21H18F3N3NaO5
Molecular Weight : 472.38






