lenvatinib mesylate
Pharmaceutical standards - Lenvatinib is approved for the treatment of differentiated thyroid cancer that is either locally recurrent or metastatic, progressive, and did not respond to treatment with radioactive iodine. It is an anti-cancer drug. Reference standards of Lenvatinib Mesylate API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.

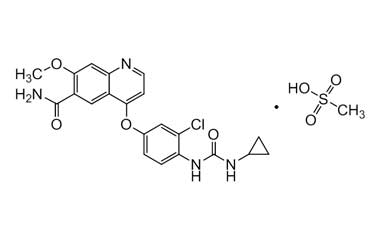
Lenvatinib Mesylate
Catalogue No.:PA 39 03000
CAS :
857890-39-2
Molecular Formula : C22H23ClN4O7S
Molecular Weight : 522.96






