Lixivaptan
Pharmaceutical standards
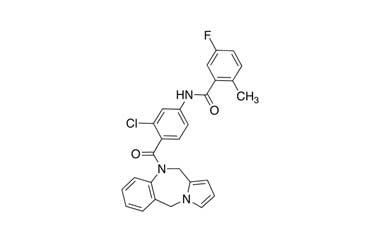
- Lixivaptan (VPA-985) is a phase III pharmaceutical being developed by Cardiokine, Inc. a specialty pharmaceutical company based in Philadelphia, PA, focused on the development of pharmaceuticals for the treatment and prevention of cardiovascular diseases. Reference standards of Lixivaptan API,and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
[pname] => Lixivaptan
[catalogue_number] => PA 65 30000
[category_ids] => ,78,75,70,76,82,
[chemical_name] =>
[weight] => 473.93
[form] => C27H21ClFN3O2
[cas] => 168079-32-1
[pslug] => 168079-32-1-lixivaptan-api-pa6530000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 65 30000
Molecular Formula : C27H21ClFN3O2
Molecular Weight : 473.93