baricitinib
Sulphur and selenium compounds
- Baricitinib was approved in February of 2017 as a second-line orally administered treatment for moderate to severe active rheumatoid arthritis in adults, either as a monotherapy or when combined with methotrexate.Baricitinib is a selective and reversible Janus kinase 1 (JAK1) and 2 (JAK2) inhibitor. Janus kinases belong to the tyrosine protein kinase family and play an important role in the proinflammatory pathway signalling that is frequently over-activated in autoimmune disorders such as rheumatoid arthritis. Reference standards of Baricitinib API, and its pharmacopeial, non pharmacopeial impurities, and stable isotopes are listed below.
stdClass Object
(
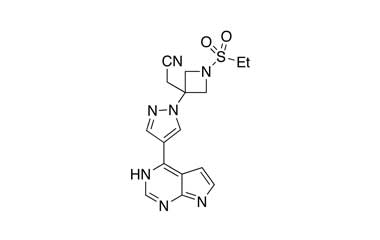
[pname] => Baricitinib
[catalogue_number] => PA 29 41000
[category_ids] => ,71,81,78,70,82,88,
[chemical_name] =>
[weight] => 371.42
[form] => C16H17N7O2S
[cas] => 1187594-09-7
[pslug] => 1187594-09-7-baricitinib-api-pa2941000
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Catalogue No.:PA 29 41000
Molecular Formula : C16H17N7O2S
Molecular Weight : 371.42
stdClass Object
(
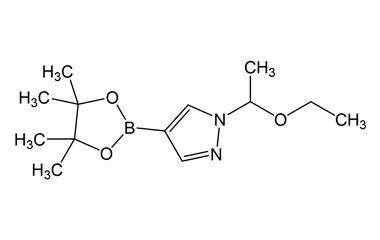
[pname] => 1-(1-Ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
[catalogue_number] => PA 29 0411017
[category_ids] => ,71,81,78,70,82,88,
[chemical_name] =>
[weight] => 266.15
[form] => C13H23BN2O3
[cas] => 1029716-44-6
[pslug] => 1029716-44-6-1-1-ethoxyethyl-4-4-4-5-5-tetramethyl-1-3-2-dioxaborolan-2-yl-1h-pyrazole-pa290411017
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
1-(1-Ethoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
Catalogue No.:PA 29 0411017
Molecular Formula : C13H23BN2O3
Molecular Weight : 266.15
stdClass Object
(
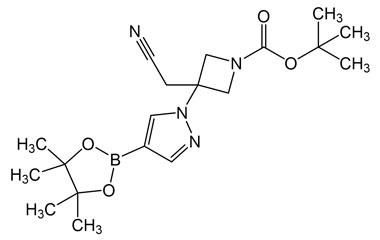
[pname] => tert-Butyl 3-(cyanomethyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)azetidine-1-carboxylate
[catalogue_number] => PA 29 0411018
[category_ids] => ,71,78,70,82,88,
[chemical_name] =>
[weight] => 388.28
[form] => C19H29BN4O4
[cas] => 1153949-15-5
[pslug] => 1153949-15-5-tert-butyl-3-cyanomethyl-3-4-4-4-5-5-tetramethyl-1-3-2-dioxaborolan-2-yl-1h-pyrazol-1-yl-azetidine-1-carboxylate-pa290411018
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
tert-Butyl 3-(cyanomethyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)azetidine-1-carboxylate
Catalogue No.:PA 29 0411018
Molecular Formula : C19H29BN4O4
Molecular Weight : 388.28
stdClass Object
(
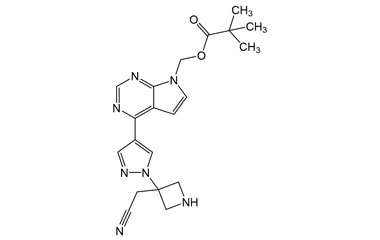
[pname] => (4-(1-(3-(Cyanomethyl)azetidin-3-yl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate
[catalogue_number] => PA 29 0411019
[category_ids] => ,71,78,70,82,88,
[chemical_name] =>
[weight] => 393.45
[form] => C20H23N7O2
[cas] => 2102104-38-9
[pslug] => 2102104-38-9-4-1-3-cyanomethyl-azetidin-3-yl-1h-pyrazol-4-yl-7h-pyrrolo-2-3-d-pyrimidin-7-yl-methyl-pivalate-pa290411019
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
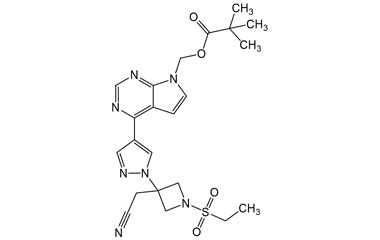
(4-(1-(3-(Cyanomethyl)azetidin-3-yl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate
Catalogue No.:PA 29 0411019
Molecular Formula : C20H23N7O2
Molecular Weight : 393.45
stdClass Object
(
[pname] => (4-(1-(3-(Cyanomethyl)-1-(ethylsulfonyl)azetidin-3-yl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate
[catalogue_number] => PA 29 0411020
[category_ids] => ,71,78,70,82,88,
[chemical_name] =>
[weight] => 485.56
[form] => C22H27N7O4S
[cas] => 1187595-90-9
[pslug] => 1187595-90-9-4-1-3-cyanomethyl-1-ethylsulfonyl-azetidin-3-yl-1h-pyrazol-4-yl-7h-pyrrolo-2-3-d-pyrimidin-7-yl-methyl-pivalate-pa290411020
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
(4-(1-(3-(Cyanomethyl)-1-(ethylsulfonyl)azetidin-3-yl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate
Catalogue No.:PA 29 0411020
Molecular Formula : C22H27N7O4S
Molecular Weight : 485.56
stdClass Object
(
[pname] => 4-Chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine
[catalogue_number] => PA 29 0411024
[category_ids] => ,78,70,82,88,
[chemical_name] =>
[weight] => 283.83
[form] => C12H18ClN3OSi
[cas] => 941685-26-3
[pslug] => 941685-26-3-4-chloro-7-2-trimethylsilyl-ethoxy-methyl-7h-pyrrolo-2-3-d-pyrimidine-pa290411024
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
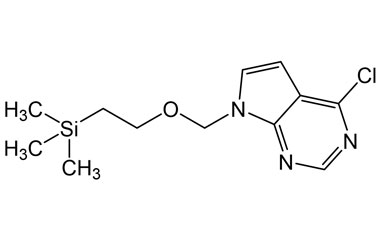
4-Chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine
Catalogue No.:PA 29 0411024
Molecular Formula : C12H18ClN3OSi
Molecular Weight : 283.83
stdClass Object
(
[pname] => 4-(1H-Pyrazol-4-yl)-7-(((2-(trimethylsilyl)ethoxy)methoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine
[catalogue_number] => PA 29 0411033
[category_ids] => ,78,70,82,88,
[chemical_name] =>
[weight] => 345.48
[form] => C16H23N5O2Si
[cas] => NA
[pslug] => 4-1h-pyrazol-4-yl-7-2-trimethylsilyl-ethoxy-methoxy-methyl-7h-pyrrolo-2-3-d-pyrimidine-pa290411033
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
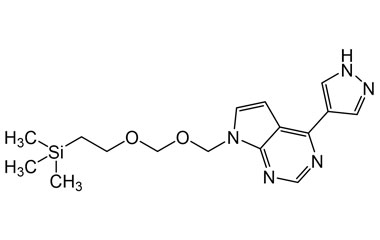
4-(1H-Pyrazol-4-yl)-7-(((2-(trimethylsilyl)ethoxy)methoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine
Catalogue No.:PA 29 0411033
Molecular Formula : C16H23N5O2Si
Molecular Weight : 345.48
stdClass Object
(
[pname] => 2-Chloro-7H-pyrrolo[2,3-d]pyrimidine
[catalogue_number] => PA 29 0411034
[category_ids] => ,78,70,82,88,
[chemical_name] =>
[weight] => 153.57
[form] => C6H4ClN3
[cas] => 335654-06-3
[pslug] => 335654-06-3-2-chloro-7h-pyrrolo-2-3-d-pyrimidine-pa290411034
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
2-Chloro-7H-pyrrolo[2,3-d]pyrimidine
Catalogue No.:PA 29 0411034
Molecular Formula : C6H4ClN3
Molecular Weight : 153.57
stdClass Object
(
[pname] => Methyl 7H-pyrrolo[2,3-d]pyrimidine-4-carboxylate
[catalogue_number] => PA 29 0411036
[category_ids] => ,71,81,78,70,82,88,
[chemical_name] =>
[weight] => 177.16
[form] => C8H7N3O2
[cas] => 1095822-17-5
[pslug] => 1095822-17-5-methyl-7h-pyrrolo-2-3-d-pyrimidine-4-carboxylate-pa290411036
[latest_product] => 0
[linkproducts] => 0
[offers_id] =>
[offers_name] =>
[offers_status] =>
[offers_start_date] =>
[offers_end_date] =>
[pageview] =>
[offers_slug] =>
[offers_product_id] =>
[offers_product_code] =>
[offers_master_id] =>
[offer_percentage] =>
[offers_product_main_cat] =>
)
Methyl 7H-pyrrolo[2,3-d]pyrimidine-4-carboxylate
Catalogue No.:PA 29 0411036
Molecular Formula : C8H7N3O2
Molecular Weight : 177.16


![2-Chloro-7H-pyrrolo[2,3-d]pyrimidine PA 29 0411034](https://www.pharmaffiliates.com/pimages/PA290411034.jpg)
![Methyl 7H-pyrrolo[2,3-d]pyrimidine-4-carboxylate PA 29 0411036](https://www.pharmaffiliates.com/pimages/PA290411036.jpg)




