Desperate hunt of Anti-Virals for COVID-19 cure

It is rightly said 'Desperate Times Call for Desperate Measures'
As cases from the coronavirusare relentlessly mounting into hundreds and thousands again and there is a hush-hush on the third Covid wave across the world, scientists are desperate to solve the deadly conundrum of COVID-19 as the world is clamouring for fast answers and solutions.
Doctors and Researchers are frantically experimenting with medicines for stroke, heartburn, blood clots, gout, depression, inflammation, AIDS, hepatitis, cancer, arthritis and even stem cells and radiation to help in fighting the deadly virus.
As the world once again reels under the massive surge of Covid-19 cases in the third wave of the pandemic, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for Merck’s Molnupiravir for the treatment of mild-to-moderate coronavirus disease in adults. Although, Molnupiravir is not a substitute for vaccination in individuals for whom COVID-19 vaccination and a booster dose are recommended.
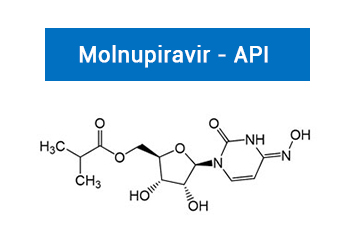
Molnupiravir an orally administered antiviral drug which was initially developed to treat influenza can significantly decrease novel coronavirus levels in hamsters, holding out promise of a pill to combat COVID-19, say researchers.
Taking into consideration the current pandemic scenario, it is believed that these findings are likely to help physicians develop appropriate treatment strategies among evolving evidence for COVID-19 management
In order to bestow a part of its contribution towards medical parlancePharmaffiliates Analytics and Synthetics Pvt. Ltd is offering a wide range of Reference material of Molnupiravir in the form of API, impurities, metabolites, intermediates, Stable isotopes etc. Here is a list of offerings which Pharmaffiliates is providing:
Reference standards of Molnupiravir
| Product Code | Chemical Name | Cas No. |
|---|---|---|
| PA 13 2380000 | Molnupiravir - API | 2492423-29-5 |
| PA 13 2381000 | 1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxyimino)-3,4-dihydropyrimidin-2(1H)-one | 3258-02-4 |
| PA 13 2381001 | 1-((2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxyimino)-3,4-dihydropyrimidin-2(1H)-one | 13491-41-3 |
| PA 13 2381002 | 1-((2S,3S,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxyimino)-3,4-dihydropyrimidin-2(1H)-one | 402725-23-9 |
| PA 13 2381003 | ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methylisobutyrate | 886538-46-1 |
| PA 13 2381004 | ((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methylisobutyrate | 886538-48-3 |
| PA 13 2381005 | ((2R,3S,4R)-3,4,5-trihydroxytetrahydrofuran-2-yl)methylisobutyrate | 2591381-21-2 |
| PA 13 2381006 | ((3aR,4R,6R,6aR)-6-(4-Amino-2-oxopyrimidin-1(2H)-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl isobutyrate | 1686124-74-2 |
| PA 13 2381007 | ((2R,3S,4R,5R)-3,4-Dihydroxy-5-((E)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl isobutyrate | 2492423-30-8 |
| PA 13 2381008 | 2',3'-Isopropylidenecytidine | 362-42-5 |
| PA 13 2381009 | Uridine-5-methylpropanoate-4-oxime | 2349386-89-4 |
There is a massive requirement of reliable pharmaceutical reference standard sources to qualify and quantify these impurities in the final product of antiviral drugs like Molnupiravir.
The reliable Reference standard provider, provides pharmaceutical standards, impurities, related substance, and stable isotopes along with a comprehensive Certificate of Analysis detailing the characterization process for the material, and ensuring its suitability for both qualitative and quantitative analysis.
The Certificate of Analysis must also take care of all the regulatory requirements.
At Pharmaffiliates, a dedicated analytical, synthesis and customer service teams go hand in hand to utilize the state-of-art infrastructure and high-end instruments with 21CFR part 11 compliance, to obtain par excellence reference standards.
In these tough times of pandemic, Pharmaffiliates shall be accepting orders, shipping product and taking inquiries by email along with implementing precautionary measures to ensure the health and safety of our people, their families, our clients and our communities.






