Levetiracetam-A Novel AED and monotherapy drug

In World's neurological disorder burden, Epilepsy has termed the fourth common disease after headache, migraine and Alzheimer's disease.
Currently, approximately two-thirds of epileptic seizures were controlled by antiepileptic drugs (AEDs) which are the main treatment method for epilepsy patients. The drugs which have been proven to have good therapeutic effects and low treatment cost are the conventional AEDs such as carbamazepine (CBZ) and sodium valproate (VPA). However, Stevens-Johnson syndrome, menstrual disorder, and memory deterioration are some major adverse events (AEs) related to these drugs which seriously affect the tolerance and compliance of patients. Although, compared with conventional AEDs, new AEDs are safer, but also more expensive.
In 2006, a monotherapy drug and a novel AED Levetiracetam (LEV) was licensed for adults and adolescents above 16 years of age with newly diagnosed focal-onset seizures.
Levetiracetam which available under the registered trademark of UCB S.A., KeppraR is the S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide.
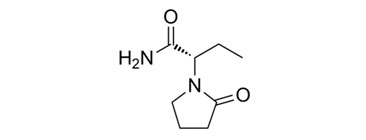
CAS No - 102767-28-2
Levetiracetam has been reported to be a broad-spectrum antiepileptic drug with a safe profile. In a recent study it was found that it is a far safer drug then its conventional counterpart sodium valproate for women of childbearing age who have migraine or epilepsy.
The above discussion proves the significance of Levetiracetam in the pharmaceutical parlance. However, fetching a high purity and high-quality drug product is an uphill task for the pharmaceutical industries and it depends upon several factors.
One major factor is pharmaceutical reference standards, which are a critical aspect in all phases of drug research, development, and commercialization.
Reference standards serve as the basis of evaluation for both process and product performance and are the benchmarks for the assessment of drug potency for patient consumption.
Now there are many impurities associated with Levetiracetam which were formed during the drug development, drug research or commercialization steps. These impurities need to be examined for their specification limit, which affects the efficacy and quality of the final drug.
List of Levetiracetam impurities are cited in this article :
| S. No | Product Name | Chemical Name | CAS No. |
|---|---|---|---|
| 1 | Levetiracetam - API | Levetiracetam | 102767-28-2 |
| 2 | Levetiracetam - Impurity A | (2RS)-2-(2-Oxopyrrolidin-1-yl)butanoic Acid | 67118-31-4 |
| 3 | Levetiracetam - Impurity B | (2Z)-2-(2-Oxopyrrolidin-1-yl)but-2-enamide | 358629-47-7 |
| 4 | Levetiracetam - Impurity C | 2-Pyridinol | 142-08-5 |
| 5 | Levetiracetam - Impurity D | (R)-Etiracetam | 103765-01-1 |
| 6 | Levetiracetam - Impurity E (Freebase) | (1R)-1-phenylethanamine | 3886-69-9 |
| 7 | Levetiracetam - Impurity E (Hydrochloride Salt) | 1-Phenylethanamine Hydrochloride | 13437-79-1 |
| 8 | Levetiracetam - Impurity G (Freebase) | (2S)-2-Aminobutanamide | 143164-46-9 |
| 9 | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide | 102767-31-7 |
| 10 | Levetiracetam - Impurity G (Hydrochloride Salt) | (S)-2-Aminobutanamide Hydrochloride | 7682-20-4 |
| 11 | Levetiracetam Racemic Mixture | Levetiracetam Racemic Mixture | 33996-58-6 |
| 12 | Levetiracetam Carboxylic Acid | Levetiracetam Carboxylic Acid | 102849-49-0 |
| 13 | N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide | N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide | 774604-48-7 |
| 14 | Levetiracetam Acid | Levetiracetam Acid | 103833-72-3 |
| 15 | L-2-Aminobutyric Acid Methyl Ester Hydrochloride | L-2-Aminobutyric Acid Methyl Ester Hydrochloride | 56545-22-3 |
| 16 | (S)-Methyl 2-(2-oxopyrrolidin-1-yl)butanoate | (S)-Methyl 2-(2-oxopyrrolidin-1-yl)butanoate | 358629-51-3 |
| 17 | (S)-2-Aminobutanoic Acid Hydrochloride | (S)-2-Aminobutanoic Acid Hydrochloride | 5959-29-5 |
| 18 | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide-d3 | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide-d3 | 1795786-85-4 |
| 19 | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide-d6 | (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide-d6 | NA |
| 20 | Levetiracetam-d3 See L331503, or L331504 | Levetiracetam-d3 See L331503, or L331504 | 1217851-16-5 |
| 21 | Levetiracetam-d6 See L331502 | Levetiracetam-d6 See L331502 | 1133229-30-7 |
| 22 | Levetiracetam-d3 (contains d0) | Levetiracetam-d3 (contains d0) | 1217851-16-5 |
| 23 | Levetiracetam-d6 (2,3,3,4,4,4-butyramide-d6) | Levetiracetam-d6 (2,3,3,4,4,4-butyramide-d6) | 1133229-29-4 |
| 24 | 2,4-Dichlorobutanoyl Chloride | 2,4-Dichlorobutanoyl Chloride | 79194-54-0 |
| 25 | (R)-N-((R)-1-Amino-1-oxobutan-2-yl)-3-((((S)-1-amino-1-oxobutan-2-yl)amino)methyl)hexanamide | (R)-N-((R)-1-Amino-1-oxobutan-2-yl)-3-((((S)-1-amino-1-oxobutan-2-yl)amino)methyl)hexanamide | NA |
To qualify and quantify these impurities in the final product of Levetiracetam, there is a massive requirement of reliable Pharmaceutical reference standard sources.
But, a particular problem is the absence of publicly available reference materials. Without the availability of this public material, no second or third party can test independently or take action. This availability is thus fundamental to the need for that medicines are manufactured according to up-to-date and relevant standards.
Reference materials are an integral component of the procedures of the private or public control specification.
The reliable Reference standard provider, provides Pharmaceutical standards, impurities, related substance, and stable isotopes of Levetiracetam along with a comprehensive Certificate of Analysis detailing the characterization process for the material, and ensuring its suitability for both qualitative and quantitative analysis.
The Certificate of Analysis must also take care of all the regulatory requirements.
At Pharmaffiliates, a dedicated analytical, synthesis and customer service teams go hand in hand to utilize the state-of-art infrastructure and high-end instruments with 21CFR part 11 compliance, to obtain par excellence reference standards.
Pharmaffiliates Analytics and Synthetics Pvt. Ltd. is a gigantic name as a leading global manufacturer and distributor of reference materials of Pharmaceutical, Phytochemicals, Agrochemicals, Deuterated products, Environmental use.







